Physical Science Solution Very Short Answer Type:
Question 1. Write down the name of an inorganic and an organic solvent.
Answer: Name of an inorganic solvent: Water.
Name of an organic solvent: Benzene.
Question 2. What is the unit of solubility?
Answer: Solubility has no unit.
Question 3. What are the sizes of the colloid particles?
Answer:
Sizes of the colloid particles: 10-5cm to 10-7 cm in diameter.
Question 4. What is occlusion?
Answer: Hydrogen gets dissolved in spongy palladium and similar metals. This phenomenon is called occlusion.
Read and Learn all WBBSE Solutions for Class 9 Physical Science And Environment
Question 5. What is TEL?
Answer: Tetra ethyl lead (TEL).
Question 6. What is the formula of Epsom salt?
Answer:
Epsom salt: MgSO4, 7H2O.
Wbbse Physical Science And Environment Class 9 Solutions
Question 7. What is SPM?
Answer: Suspended particulate matter (SPM).
Question 8. What do you mean by smog?
Answer: Smog is fog-containing smoke.
Question 9. How does the solubility of sodium sulfate change with temperature? Or, Give an example of a substance whose solubility first increases and then decreases with the increase in temperature very slowly.
Answer: The solubility of sodium sulfate first increases up to 40°C and then it slowly decreases to a little extent up to 80°C.
Question 10. Give an example of a neutral drying agent.
Answer: Anhydrous magnesium sulfate (MgSO4).
WBBSE Class 9 solution chapter solutions
Question 11. Give an example of an acidic drying agent.
Answer: Phosphorus pentoxide ( P2O5) is an acidic drying agent.
Question 12. Give an example of a basic drying agent.
Answer: Quick lime (CaO) is a basic drying agent.
Question 13. Give an example of aerosol.
Answer: One aerosol-fog.
Question 14. Give an example of one natural colloid.
Answer: Gelatin or Glue is a natural colloid.
Question 15. What is mist?
Answer: Aerosol is formed by the suspension of liquid particles (water, acid, etc.) in air.
Wbbse Physical Science And Environment Class 9 Solutions
Question 16. Name two diseases caused by SPM.
Answer: Asthma and lung cancer.
Question 17. Solubility of which substance does not change appreciably with an increase of temperature?
Answer: Sodium chloride. (NaCl)
Question 18. What is Mother liquor?
Answer: In the process of crystallisation, the saturated solution left after the separation of crystals is called Mother liquor.
Question 19. The solubility of A at °C is x”. What does it mean?
Answer: It means x gram of A can produce a saturated solution when dissolved in 100 grams of solvent at t°C.
Question 20. Give an example of a compound whose solubility in water decreases with an increase in temperature.
Answer: The solubility of slaked lime decreases with an increase in temperature.
Question 21. What are the components of a colloidal solution?
Answer:
Components of a colloidal solution :
(1) Dispersed phase
(2) Dispersion medium.
Question 22. Write down the name of a substance whose solubility decreases with an increase in temperature.
Answer:
Solubility decreases with a temperature increases: Calcium sulphate (CaSO4).
Question 23. Which gas is dissolved in soda water?
Answer: Carbon dioxide (CO2).
Question 24. Write down the name of a substance whose solubility increases tremendously with an increase in temperature.
Answer:
Solubility increases tremendously with an increase in temperature: Potassium nitrate (KNO3).
Question 25. Give one example of a salt having water of crystallisation.
Answer:
Example of a salt having water of crystallisation: Blue vitriol (CuSO4. 5H2O).
Question 26. What is the effect of increase in pressure on the solubility of a solid in water?
Answer: Increase in pressure does not cause any change of solubility of a solid in water.
Wbbse Physical Science And Environment Class 9 Solutions
Question 27. Name a deliquescent substance.
Answer:
Deliquescent substance : Calcium chloride (CaCl2).
Question 28. Mention one particulate matter in air that causes its pollution.
Answer:
Particulate matter in air that causes its pollution: Fine particles of dust.
Question 29. Is solution a mixture or compound?
Answer: Solution is a mixture.
Question 30. Why does edible salt (NaCl) become moist in the rainy season?
Answer: Edible salt (NaCl) becomes moist in the rainy season due to the presence of deli quescent MgCl2, 6H2O as impurity in it.
Question 31. Name an efflorescent substance.
Answer:
Efflorescent substance: Washing soda (Na2CO3.10H2O).
Question 32. What happens if a saturated solution is cooled
Answer: The cooled solution sheds the excess solute as crystals.
Question 33. What is the effect of increase in pressure on the solubility of a gas in water ?
Answer: Increase in pressure increases solubility of a gas.
Solution WBBSE Class 9 solutions with answers
Question 34. What is solute?
Answer:
Solute: The substances which are present in smaller quantities and get dissolved are called solutes.
Question 35. What is solvent?
Answer:
Solvent: The medium in which the solutes are uniformly dispersed through dissolution is known as the solvent.
Question 36. What is a homogeneous mixture?
Answer:
Homogeneous mixture: A homogeneous mixture is one in which the constituent substances are so intimately mixed that even a very close examination cannot distinguish any surface of separation between them.
Wbbse Physical Science And Environment Class 9 Solutions
Question 37. What is a heterogeneous mixture?
Answer:
Heterogeneous mixture: A heterogeneous mixture is one in which the particles of the constituent substances of a mixture are distinctly distinguishable.
Question 38. What is a colloidal solution?
Answer:
Colloidal solution: A colloidal solution is a stable heterogeneous solution consisting of finely divided particles of a substance (of the size of 10-5 cm to 10-7cm in diameter) uniformly dispersed in a continuous medium.
Question 39. What are dispersed phase and dispersion medium?
Answer:
Dispersed phase and dispersion medium: The finely divided Particles are called the dispersed phase and the continuous medium is called the dispersion medium.
Question 40. What is suspension?
Answer:
Suspension: The particle in suspension state is 10-4 cm in diameter.
Question 41. What is true solution?
Answer:
True solution: The particle in true solution is 10-8 cm in diameter
Question 42. What is sol?
Answer:
Sol: It is a colloidal system in which a solid is dispersed in a liquid
Example: Paints.
Question 43. What is emulsion?
Answer:
Emulsion: It is a colloidal system in which a liquid is dispersed in another liquid .
Example: Milk
Question 44. What is Gel?
Answer:
Gel: It is a colloidal system in which a liquid is dispersed in a solid
Example: Fruit jellies, cheese, etc.
Question 45. What is aerosol?
Answer:
Aerosol: It is a colloidal system having a solid or a liquid dispersed in a gas
Example: Fog, smoke, clouds, etc.
Question 46. Define Lyophilic colloids.
Answer:
Lyophilic colloids: These are the substances which pass readily into the colloidal state whenever mixed with a suitable solvent
Protein, starch, etc.
Question 47. Define Lyophobic colloids.
Answer:
Lyophobic colloids: These are the substances which do not yield colloidal solutions on mere shaking with a liquid.
Example: Gold, silver, Fe (OH)3, As2 O3
Question 48. What are positive sols?
Answer:
Positive sols: These are the sols which carry positive charge on the dispersed phase particles.
Example: Sols of Fe(OH)3, Al(OH)3 , etc.
Question 49. What are negative sols?
Answer:
Negative sols: These are the sols which carry negative charge on their particles).
Example: Sols of Cu, Ag, Au, As2,S3, etc.
Question 50. What are multi-molecular colloids?
Answer:
Multi-molecular colloids: These are the colloids in which the individual particles consist of aggregates of atoms of small molecules having molecular size less than 10-7 cm in diameter
Example: Sols of gold atoms, platinum sol.
Question 51. What are macro-molecular colloids?
Answer:
Macromolecular colloids : These are the colloids in which the size of the particles of the dispersed phase are of the order of colloidal dimensions.
Example: Sol of starch, cellulose
Question 52. What is peptisation?
Answer:
Peptisation: It is the process of converting precipitates into colloidal state by adding small amount of a suitable electrolyte.
Question 53. What is dialysis?
Answer:
Dialysis : It is the process of separating substances in colloidal state from those present in ionic states with the help of semipermeable membrane.
Question 54. What is Tyndall effect?
Answer:
Tyndall effect: It is the scattering of light from the surface of colloidal particles and a beam of light passed through a colloidal solution becomes visible as a bright streak. The illuminated path is called the Tyndall effect.
Question 55. What is Brownian movement?
Answer:
Brownian movement: This is a ceaseless, erratic, and random motion of colloidal particles as a result of their bombardment by the molecules of dispersion medium.
WBBSE Class 9 Physical Science solution notes
Question 56. What is electrophoresis?
Answer:
Electrophoresis: It is the movement of charged colloidal particles, under the influence of an electric field, towards the oppositely charged electrodes.
Question 57. What is coagulation?
Answer:
Coagulation: It is the phenomenon of changing of colloidal state to a suspended state. It can be brought about by adding an electrolyte to a colloidal solution.
Question 58. What is Hardy Schulze’s rule?
Answer:
Hardy Schulze rule: It states the greater the valency of the coagulating ion added, the greater is its power to coagulate.
Question 59. What is gold number?
Answer:
Gold number: It is the weight in milligrams of a protective colloid which prevents the coagulation of 10 mi of a given gold sol on adding 1 ml of 10% solution of sodium chloride.
Question 60. What is solubility?
Answer:
Solubility: Solubility of a given solute in a solvent is defined as the weight in grams of the solute dissolved in 100 grams of the solvent so as to saturate the solution at a given temperature.
Question 61. What is Henry’s law?
Answer: At a definite temperature the solubility of a gas in a particular solvent is proportional to the pressure applied on the gas. This is Henry’s law.
Question 62. What are crystals?
Answer:
Crystals: Crystals are homogeneous solid particles (big or small) with definite geometric shape, and are bounded by plane surfaces meeting at sharp edges.
Question 63. What is crystallisation ?
Answer:
Crystallisation : Crystallisation is a process by which crystals of a substance are obtained from its solution.
Question 64. What is water of crystallisation ?
Answer:
Water of crystallisation : When a crystal is formed from the aqueous solution of a substance then one or more fixed number of water molecules associated with each molecules of that substance by chemical bonding are called water of crystallisation.
Question 65. What are hydrated crystals?
Answer:
Hydrated crystals: Crystals having the water molecules associated with them are called hydrated crystals.
Question 66. Which is the solvent in the following binary solutions?
(1) Sugar and water
(2) 70 ml of alcohol and 30 ml water
(3) Soda water.
Answer:
(1) Water
(2) Alcohol
(3) Water (CO2 , is solute).
Question 67. Which is the solute in the-following solutions
(1) Hydrogen chloride and water
(2) 80 ml of nitrogen and 20 mI hydrogen.
Answer: (1) Hydrogen chloride
(2) Hydrogen.
Question 68. If 15 g of a saturated solution of sodium chloride at 20°C, leaves a solid residue of 3 g, when evaporated to dryness, calculate the solubility of NaCl at that temperature.
Answer:
Given
If 15 g of a saturated solution of sodium chloride at 20°C, leaves a solid residue of 3 g, when evaporated to dryness,
Wt. of water in solution = 15 − 3 = 12 g.
So 12 g H2O dissolves 3 g of solid.
∴ 100 g H2O dissolves \( \frac{3}{12} \times 100\)g of solid = 25 g.
∴ Solubility in % (w/w) = 25.
Question 69. A 200 mi saline solution contains 1.8 g of NaCl. Find the % w − v of the solution.
Answer:
Given
A 200 mi saline solution contains 1.8 g of NaCl.
% w-v=\(\frac{w}{V} \times 100\) = 0.9% (w in ‘g’ and V in ml taken)
Question 70. 50.9 of sugar is dissolved in water to prepare a 2.50-litre solution, calculate its concentration in grams per litre.
Answer:
Given
50.9 of sugar is dissolved in water to prepare a 2.50 litre solution
Mass of sugar = 50 g.
Volume of solution = 2.50 litre
∴ g/l Concentration\(=\frac{50 \mathrm{~g}}{2.5001}\) = 20 gl-1
Question 71. 8 g of NaOH is dissolved in water to prepare 1500 mi solution. Calculation its molar concentration. (NaOH = 40)
Answer:
Given
8 g of NaOH is dissolved in water to prepare 1500 mi solution.
Weight of NaOH = 8g.
Volume of solution = 1500 ml.
8x 1000
Molarity of the solution \(=\frac{8 \times 1000}{1500 \times 40}\)=0.13 mol.L-1
Question 72. At 20°C, 5 g of a salt is dissolved in 10 g of water and the solution is saturated. What will be the solubility of salt at that temperature?
Answer:
Given
At 20°C, 5 g of a salt is dissolved in 10 g of water and the solution is saturated
Mass of solute = 50 g and mass‘of the solvent = 10 g.
So, solubility \(=\frac{\text { wt. of solute }}{\text { wt. of solvent }} \times 100\)
=\(\frac{5}{10} \times 100\)
=50
Solution 2 Marks Questions And Answers:
Question 1. Why does a finely powdered solute dissolve much quicker in a liquid solvent than a lump of equal weight of the same solute?
Answer: Dissolution of a solid solute in a liquid solvent takes place from the surface of the solid exposed to the liquid solvent, so, if larger surface area of the solid be exposed to the liquid, greater rate of dissolution takes place. For this reason, a finely powered solute dissolves much quicker in a liquid solvent than a lump of equal weight of the same solute.
Question 2. What are concentrated and dilute solutions?
Answer:
Concentrated and dilute solutions
A solution that contains a relatively small amount of solute compared to the amount of solvent is referred to as dilute, while a solution that contains a relatively large amount of solute is referred to as a concentrated solution.
Question 3. Can there be solvents other than water?
Answer: Liquids other than water may also act as solvents. Sulfur is insoluble in water but dissolves in carbon disulfide forming a clear solution. Water is incapable of dissolving camphor, waxes, fats, and oils but chloroform, ether, alcohol, benzene, etc. are good solvents for these substances.
Question 4. What do you mean by ‘dispersed phase’ and ‘dispersion medium’?
Answer:
Dispersed phase: The component of the colloid present in small amounts and which behaves like a solute in a solution is called dispersed phase.
Dispersion medium: The component of colloid present in excess amount and which behaves like a solvent in a solution is called dispersion medium.
Question 5. Define the unit of concentration of the solution in percentage.
Answer:
Concentration of solution in percentage
If V ml of the solution contains W g of the solute, the percentage of the solution is \(\frac{W}{V} \times 100 \%\).
Question 6. Define the unit of concentration of the solution in grams per liter.
Answer:
The concentration of solution in grams per liter:
Its unit is g/l. When 1 litre (1000. ml) of the solution contains w grams of the solute, its strength is w g/1l.
Question 7. What happens when a saturated solution of a substance at 100°C is cooled to 60°C?
Answer: The solution becomes super-saturated and an amount of solid substance which is in excess than can be present in a saturated solution at 60° will separate out.
Question 8. What is meant by 2% NaCl solution?
Answer:
2% NaCl solution
2% NaCl solution means that 100 milliliters of the given solution of sodium chloride in water contains 2 gm of dissolved NaCl.
Question 9. What is meant by a concentration of 20 gm/liter of a solution?
Answer:
The concentration of 20 gm/liter of a solution
A concentration of 20 ml liter means that 1 liter, ie., 1000 milliliters of the given solution contains 20 gm of dissolved solute.
Question 10. What is the difference between aqueous and non-aqueous solutions?
Answer:
The difference between aqueous and non-aqueous solutions
True solution of any solute in water is called an aqueous solution, e.g. vinegar. The true solution in any solvent except water is called non-aqueous solution, e.g. amino acids dissolved in acetone.
Important questions on solution WBBSE Class 9
Question 11. What is an anhydrous substance or salt?
Answer:
Anhydrous substance or salt
A hydrated salt which completely loses its water of crystallisation or a substance that-does not exist in hydrated state can be called anhydrous.
Question 12. What is hydrated substance or hydrated salt?
Answer:
Hydrated substance or hydrated salt
A substance (or salt) which contains certain fixed number of water molecules, bound to its one molecule, into loose chemical bond, is called hydrated substance or hydrated salt.
Question 13. Why are anhydrous calcium chloride and phosphorus pentoxide kept in air tight bottles?
Answer: Anhydrous calcium chloride and P2O5 are deliquescent substances and become liquid by absorbing moisture from air if they come in contact with air. That is why, these substances are to be stored in air tight bottles.
Question 14. Giving example, state the use of suspension in our daily life.
Answer: Many substances insoluble in dispersing medium but which form suspension temporarily are used for analytical purposes. For example, sparingly soluble barium sulphate dispersed in water is an opaque medium and is used for taking intestinal X-ray photograph.
Question 15. State the observation on the basis of which you may identify a suspension.
Answer: If the particles in a heterogenous and opaque solution can be seen by the naked eye or under an ordinary microscope and get settled on keeping, then it is a suspension.
Question 16. What do you mean by solution ? Give an example.
Answer:
Solution:
A homogeneous mixture of solid substance, in liquid (usually water) having same physical properties in every part of it is called the solution.
Example: When small quantity of sugar is added in a glass full of water, then the sugar will disappear in the water on stirring. The product so obtained is called the solution of sugar in water.
Question 17. What are solvent and solute? Give example.
Answer:
Solvent: A liquid (say water) which allows a solid to dissolve in it, so as to form solution, is called solvent.
Solute: A solid which dissolves (disappears) in a liquid (say water) to form a solution is called solute.
Examples: When sugar dissolves in water to make a solution, then water is called solvent and the sugar is called solute.
Question 18. How can it be known with a single experiment whether a solution is saturated or not?
Answer: Take some waier in a beaker and dissolve some sugar into it. Stir well to make it a clear solution. Now add some more sugar into the solution and stir well the solution. If the sugar dissolves completely at the room temperature, then the solution is unsaturated. If the sugar stops dissolving at the same temperature and settles down in the bottom of the beaker, then the solution is saturated at that temperature.
Question 19. What is a colloidal solution? Give example.
Answer:
Colloidal solution
A solution in which the size of solute particles is larger than that of the molecules of true solution but less than the suspended particles, it is said to be a colloidal solution.
Example: Very finely ground sulphur when mixed with water, filtration does not remove all the sulphur particles. Some particles of sulphur are fine enough to remain permanently mixed with the water, it is called colloidal solution. When the liquid is examined by an ultramicroscope, it can be seen that sulphur particles are in rapid random motion at room temperature. This movement of colloidal particles is known as the Brownian movement.
Question 20. What are the suspended particulate matter in air ?
Answer :
Suspended particulate matter in air
The finely divided solid or liquid particles suspended in air are called particulates. Some of the examples of
Particulates present in the air are Dust, smoke, fumes, mist, flyash. Particulates can be solids or liquids. For example, dust, smoke and fumes are solid particulates, whereas mist and spray are liquid particulates. Other particulates in the air are pollen grains, Bacteria, Fungi, Viruses, fine sand particles, pesticides, and dust of several industrial wastes.
Some particulates are very small, fe particles and have a diameter of less than one micrometer such as smoke, fumes, aerosols, etc. Large solid particles such as dust, mist,etc. have a diameter more than-one micrometer.
Question 21. Define solubility of a solute.
Answer:
Solubility of a solute
The solubility of a solute in a solvent at a particular temperature is the number of grams of the solute necessary to saturate 100 gms of the solvent at that temperature.
Thus, solubility of a solute at t°C\(=\frac{\text { mass of solute }(\mathrm{g})}{\text { mass of solvent }(\mathrm{g})} \times 100\).
For example The solubility of potassium nitrate in water at 20°C is 32. It means that at 20°C, 32 gm of potassium nitrate (maximum amount) can be dissolved in 100 gm of water to make a saturated solution of it.
Question 22. On what factors does the solubility of substances depend?
Answer :
Different substances have different solubilities at different temperaturés.
The difference in solubilities of substances depends upon :
(1) Nature of solute
(2) Nature of solvent used
(3) Temperature
(4) Pressure.
Question 23. What are solubility curves? State their application.
Answer:
Solubility curves
The graph drawn for the solubility of a substance at different temperatures is called the solubility curve. In the solubility curve graph, temperature is plotted on the x-axis, and the solubility of the substance in 100 gm of water on the y-axis. Some uses of solubility curves are:
(1) To find the solubility of a given substance at some particular temperature.
(2) To compare the solubility of different substances at a given temperature.
Question 24. What are crystals and crystallization?
Answer:
Crystals and crystallization
When a saturated solution available at a particular temperature is cooled to a lower temperature, then some of the dissolved solutes will separate out in the form of a definite geometrical shape called a crystal. The process of forming crystals is called crystallization. Crystals are homogeneous solids bounded by a plane surface at definite angles to one another and having a definite geometric shape.
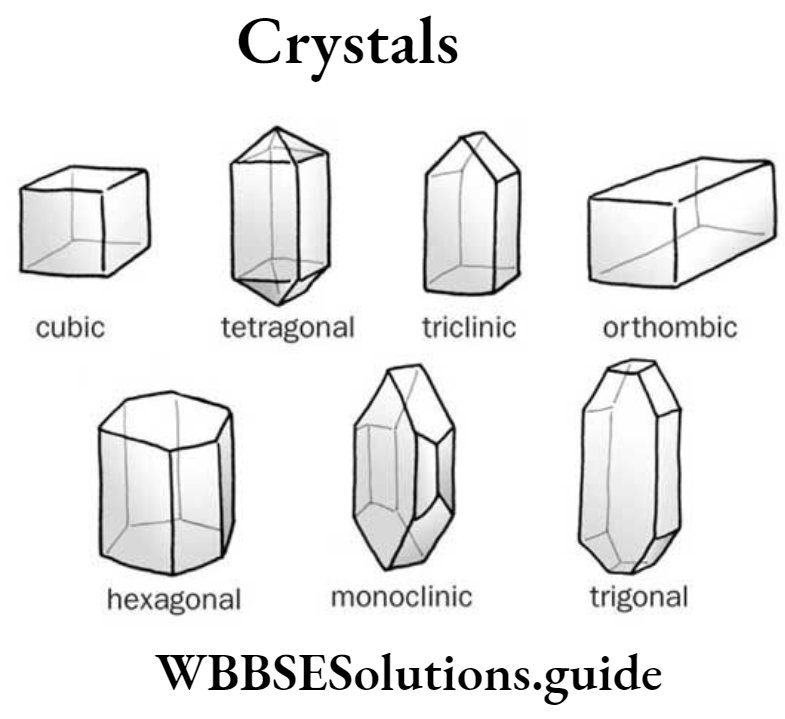
Question 25. State the physical properties of crystals with examples.
Answer :
The physical properties of crystals of a substance are :
(1) Crystals are solid particles having a definite regular shape.
(2) Crystals are bounded by flat surfaces at definite angles to each other.
(3) Crystals are the purest form of substances. The impurities dissolved in the saturated solution will be separated.
Examples of some crystals :
Question 26. What is Water of Crystallization?
Answer:
Water of Crystallization
Crystals of some substances bind a certain fixed number of water molecules while separating from their saturated solution on cooling. These fixed numbers of water molecules attached to the crystals of the substances determine the shape and colour of the crystals, called water of crystallization. The fixed number of water molecules form a loose chemical bond.
For example :
Copper sulphate crystals – CuSO4.5H2O
Magnesium sulphate – MgSO4.7H2O
Washing soda or hydrated sodium carbonate – NaCO3.10H2O
Question 27. What are hygroscopic substances? Give example.
Answer:
Hygroscopic substances
A substance which when placed in open air, absorbs moisture from it, but does not change its state, is called hygroscopic substance.
Example: Anhydrous sodium carbonate (Na2 CO3).
Anhydrous quick lime (CaO).
Concentrated sulphuric acid (H2SO4).
WBBSE Class 9 solved exercises on solution
Question 28. What are drying agents: Give some examples of drying agents.
Answer:
Drying agents
The substances which can absorb moisture from other substances without chemically reacting with them, are called drying agents.
Examples of drying agents:
Anhydrous Calcium chloride (CaCl2),
Quick lime (CaO),
Phosphorus pentoxide (P2 O5 ),
Concentrated sulphuric acid (H2SO4).
Silica gel, etc.
Question 29. What is the cause of efflorescence? Give one example of an efflorescent substance.
Answer:
Cause of efflorescence: Efflorescence happens only when the vapor pressure within the hydrated crystal at ordinary temperature is greater than the vapour pressure of the atmosphere. These hydrated crystals are called efflorescent substances.
Example: Na2CO3, 10H2O.
Question 30. What is the cause ‘of deliquescence? Give one example of a deliquescent sub- stance.
Answer:
Cause of deliquescence: Deliquenscence occurs when the vapour pressure of water in the deliquescent substance is less than the vapour pressure in the atmosphere at ‘ordinary temperature.
Example: CaCl2.
Question 31. Why does the colour of sky appear to be blue?
Answer: Colour of sky appears to be blue due to the scattering of blue light by dust particles along with water suspended in air.
Question 32. What is fire foam?
Answer:
Fire foam: Carbon dioxide froth made. mixing a solution of sodium bicarbonate and alum is called fire foam. It is used in fire extinguishers. A protective gold such as glue or dextrin is added to stabilize the foam.
Question 33. What do you understand by (1) Aqueous solution and (2) Non-request solution? Give example.
Answer:
(1) Aqueous solution: A solution obtained by dissolving a solute in water is called aqueous solution.
Example: A solution prepared by dissolving 5 gm of common salt in water is an aqueous solution of salt and is written as NaCl (aq).
(2) Non-Aqueous solution: A solution in which the solvent is any liquid except water is called a non-aqueous solution. Thus, a solution of sugar in alcohol is an example of nonaqueous solution.
Question 34. What are the differences between suspension, colloidal, and true solution in respect of the size of their solute particles?
Answer:
The differences between suspension, colloidal, and true solution in respect of the size of their solute particle
The particles of a solute forming a true solution of molecular size (1-10A) are invisible and do not settle on standing. The solute forming suspension are much bigger (>2000A) and visible, if not by the naked eye, at least under a microscope. The particles seitle down within a short time. But the sizes of the particles (10A-2000A) forming colloidal solution are bigger than molecules but too small to be visible under a most powerful microscope.
Question 35. The solubility of urea is 109.04 g/100 cc in water at 20°C. A solution is formed by dissolving 500 g urea in 1 liter water. State the nature of the solution formed.
Answer:
Given
The solubility of urea is 109.04 g/100 cc in water at 20°C. A solution is formed by dissolving 500 g urea in 1 liter water.
The solution formed is unsaturated since it contains lesser amount of the solute than that can be dissolved in the given volume of water at 20°C.
The solution contains\(\frac{500 \mathrm{~g}}{1000 \mathrm{c} . \mathrm{c}} \times 100\)= 50 g urea per 100 c.c. water.
Question 36. The concentration of an aqueous solution of NaCl is 2% wiv. Express the strength of the solution in g militre.
Answer:
Given
The concentration of an aqueous solution of NaCl is 2% wiv.
100 millilitres of the given water solution contains 2 gm of NaCl.
∴ 1000 milliliter of the given water solution contains \(\frac{2 \times 1000}{100}\)=g
∴ The strength of the solution = 20 g/litre.
Question 37. How many grams of NaCl will be necessary to prepare 500 mi of 5% NaCl?
Answer:
5% NaCl contains 5 grams of NaCl per 100 mi of the solution.
So the number of grams of NaCl required to prepare 500 ml of 5% NaCl solution
\(=\frac{5}{100}\)= 500 = 25 gm.
Question 38. 15.20 gm of a saturated solution of cane sugar at 20°C contains 10.28 g of cane sugar. Calculate the solubility of cane sugar at 20°C.
Answer:
Given
15.20 gm of a saturated solution of cane sugar at 20°C contains 10.28 g of cane sugar.
10.20 gm of sugar is present in 15.20 g of the solution.
Amount of water present in 15.20 g of the solution
= (15.20 -10.20) g= 5g.
Now, 5 g of water contains 10.20 g of sugar.
100 g of water contains\(\frac{10.20 \times 100}{5}\)=204 g of sugar.
Solubility of sugar in water at 20°C = 204.
Question 39. 2.5 g of sodium chloride is dissolved in water and the volume is made up to exactly 250 cm3. Determine the percentage strength of this solution.
Answer:
Given
2.5 g of sodium chloride is dissolved in water and the volume is made up to exactly 250 cm3.
strength in weight by volume (w/v) is the number of solute particles presenting in 100 c.c. of the solution.
∴ Percentage strength of the sodium chloride solution
(% by w/v)\(=\frac{2.5 \times 100}{250}\)=1
Question 40. 1. 5 gm of sodium chloride is dissolved in water and the volume is made up to exact 250 cm3. Determine the percentage strength of this solution.
Answer:
Given
5 gm of sodium chloride is dissolved in water and the volume is made up to exactly 250 cm3.
5 gm of solute is present in 250 c.c. of the given solution. So the weight-volume (w/v) The percentage strength of the given solution will be
\(\frac{5}{250}\) = 250x 100 = 2% (w/v).
Question 41. Why does effervescence occur when a soda water bottle is opened?
Answer: Soda water is a solution of carbon dioxide in water prepared. under high pressure. For this reason when a bottle of soda water is opened, i.e., pressure on the liquid content in the bottle is reduced, effervescence or rising bubbles occur. These effervescences form as a part of dissolved CO gas escapes through the liquid.
Question 42. Why does clear line water turn miiky when heated?
Answer: Since solubility of slaked lime [Ca(OH)2] decreases with increase in temperature, clear lime water, which is an aqueous solution of Ca(OH)2 or slaked lime, turns milky when heated.
Reason : Due to decrease of solubility at a higher temperature, the excess undissolved slaked ‘lime remains in suspension for which the solution appears milky.
Question 43. Give a simple experiment with which a given solution can be identified whether it is unsaturated or saturated or supersaturated.
Answer: A little quantity of solute of the given solution is added to the solution; if the solute dissolves instantly, the given solution slowly increases, the given salution is supersaturated.
Question 44. Calculate the molality of a solution prepared by dissolving 10.5 g of NaCl in 250 g of H2O.
Answer:
Molarity (m)=\(\frac{\mathrm{n}(\mathrm{mol})}{\mathrm{w}(\mathrm{kg})}\)=\(\frac{\left(10.5 \mathrm{~g} / 58.44 \mathrm{~g} \mathrm{~mol}^{-1}\right)}{\left(250 \mathrm{~g} / 1000 \mathrm{~g} \mathrm{~kg}^{-1}\right)}\)
=0.719 mol Kg-1
We report this molality as 0.719 m NaCl solution.
Question 45. 36 g of saturated solution of sodium chloride at 20°C, when evaporated to dryness, leaves a solid residue of 9 g. Calculate the solubility of sodium chloride.
Answer:
Given
36 g of saturated solution of sodium chloride at 20°C, when evaporated to dryness, leaves a solid residue of 9 g.
Weight of water in solution = (36 − 9) = 27 g.
27 g water dissolves 9 g NaCl
∴ 100 g water dissolves\(=\frac{9}{27} \times 100\)=33.33
Solubility of NaCl in water at 20°C=33.33
Question 46. Find the weight of potassium nitrate which is to be taken to prepare 60 g pure crystals from its saturated solution at 80°C. The solubility of potassium nitrate at 80°C is 140 g. and at 25°C is 100g.
Answer:
Solubility at 80°C = 140, Solubility at 25°C = 100.
Amount of crystals obtained when the solution is cooked to 25°C = 140 −100 = 40g.
To obtain 40g KNO3 crystals potassium nitrate was taken = 140 g.
To obtain 60g KNO3 crystals potassium nitrate to be taken \(=\frac{140}{40} \times 60\)=210g.
Question 47. At 20°C, in 12 g of a saturated solution of sugar, 2 g of sugar is dissolved. What will be the solubility of sugar ?
Answer:
Solution:
Given
At 20°C, in 12 g of a saturated solution of sugar, 2 g of sugar is dissolved.
Mass of solution = 12 g, mass of sugar (solute) = 2 g.
So, the mass of solvent (water) = 12 − 2 = 10.
So, solubility \(=\frac{\text { wt. of solute }}{\text { wt. of solvent }} \times 100\)=\(\frac{2}{10} \times 100\)= 20.
WBBSE Class 9 Physical Science types of solutions solutions
Question 48. At 20°C, the solubility of NaCl is 36. At this temperature in a saturated solution of the salt 400 g water is present. What amount of salt is obtained on evaporation of the solution?
Answer:
Given
At 20°C, the solubility of NaCl is 36. At this temperature in a saturated solution of the salt 400 g water is present.
Mass of solvent = 400 g, and solubility = 36.
So, solubility\(=\frac{\text { wt. of solute }}{\text { wt. of solvent }} \times 100\) or, 36\(=\frac{\text { wt. of solute }}{400} \times 100\)
So, wt. of solute\(=\frac{400 \times 36}{100}\)=144g.
Question 49. At 30°C, the solubility of salt is 40. What amount of water is required to form a solution with 30 g of salt? What will be the amount of the solution?
Answer:
Given
At 30°C, the solubility of salt is 40. What amount of water is required to form a solution with 30 g of salt?
Mass of solute = 30, solubility = 40
So, solubility\(=\frac{\text { wt. of solute }}{\text { wt. of solvent }} \times 100\) or, 40\(=\frac{30}{\text { wt. of solvent }} 100\)
∴ wt. of solvent \(=\frac{30 \times 100}{40}\)=75g.
Amount of water required = 75 g.
And the amount of the solution = wt. of solute + wt. of solvent = (30 + 75) = 105 g.
Question 50. A solution contains 2 g of the solute in 25 mi of the solution at 25°C. What will be the gram per liter strength of the solution?
Answer:
Given
A solution contains 2 g of the solute in 25 mi of the solution at 25°C.
25 ml contains 2 g of solute.
1 ml contains \(\frac{2}{25}\) g of solute.
1 l=100 ml contains\(\frac{2 \times 1000}{25}\)=2×40 g of solute=80
Question 51. A solution contains 5 g mole of a solute in 250 ml-of the solution at 27°C. What will be the mol I” strength of the solution?
Answer:
Given
A solution contains 5 g mole of a solute in 250 ml-of the solution at 27°C.
250 ml contains 5 g mole of solute.
1 ml contains \(\frac{5}{250}\)mole of solute.
1 l= 1000 ml contains \(\frac{5 \times 1000}{250}\) g mole of solute
=5 x 4 g mole of solute = 20 g mole of solute.
So, strength = 20 mol L-1 .
Question 52. A solution contains 8 g of NaCl in 25 g of water at 30°C. What will be the weight percentage strength of the solution?
Answer:
Given
A solution contains 8 g of NaCl in 25 g of water at 30°C.
25 g water contains 8 g of the solute
1 g water contains\(\frac{8}{25}\) of the solute
or, 100g water contains \(\frac{8 \times 100}{25}\) g of the solute
= 8x 4 g of the solute
= 32 g of solute.
So, the strength = 32%.
Question 53. A solution has a strength 40 mol L-1 at 30°C. What will be the amount of solute present in 250 ml of that solution at 30°C?
Answer:
Given
A solution has a strength 40 mol L-1 at 30°C.
1 l= 1000 ml contains 40 moles of solute
1 ml contains\(\frac{40}{1000}\) moles of solute
250 ml contains\(\frac{40\times 250}{1000}\) moles of solute
= 10 moles of solute
So, no. of gram mole of solute present = 10.
Question 54. At 27°C a solution has a strength of 35%. What will be the amount of the solute in 50 g of water?
Answer:
In 100 g water, amount of solute present = 35 g.
1 g water, amount of solute present =\(\frac{35}{100}\)
∴ In 50 g water, amount of solute present =\(\frac{35\times 50}{100}\)
So, the amount of solute = 17.5 gram.
Question 55. At 25°C, the strength of a solution is 60 g/l. What will be the amount of solute in 250 ml of that solution?
Answer:
In 1000 mi solution amount of solute = 60 g.
1 ml solution amount of solute =\(\frac{60}{1000}\) g.
In 250 ml solution amount of solute = \(\frac{60\times 250}{1000}\) g
= 15 g.
So, the amount of solute = 60 g.
Solution 3 Marks Questions And Answers:
Question 1. Given below is a list of some colloids. Make a list selecting them based on dispersing medium. Smoke, fog, cloud, milk, jelly, cheese, blood, paint, shaving cream, milk of magnesia.
Answer:
Fog, Cloud → Air (gas)
Smoke → Air (gas)
Shaving cream → Liquid (water)
Blood → Liquid (water)
Paint → Liquid (water)
Milk of Magnesia → Liquid (water)
Milk → Liquid(water)
Jelly, Cheese → Solid
Question 2. Classify the following into true solution, colloid, and suspension
(1) Lime water
(2) Aerated water
(3) Milk
(4) Sugar in water
(5) Writing ink,
(6) Blood
(7) Butter
(8) Muddy water
(9) Calcium carbonate in water
(10) Liquid
Answer:
True solutions: Lime water, aerated water, sugar in water.
Colloids: Milk, butter, blood, writing ink, liquid adhesive.
Suspension: Muddy water, calcium carbonate in water.
Question 3. State the different types of solutions by combining different states of matter.
Answer:
We can classify the different types of solution into :
(1) Solids in liquid
(2) Liquid in liquid
(3) Gases in liquid
Examples : The solution of solids in liquids are – solution of sugar and water, solution of potassium nitrate in water, etc.
The solution of liquids in liquids are – solution of milk and water, solution of water and glycerine, etc.
The solution of gases in liquids are – water and air, water and carbon dioxide (soda water or aerated water).
Question 4. State the differences between solution and colloids.
Answer :
The main differences between true solutions and colloids are given below :
| Solution (or true solution) | Colloid (or colloidal solution) |
| 1. The average size of solute particle in a true solution is 10-8 cm. | 1. The size of solute particles in a colloid is between 10-7 cm and 10-5 cm. |
| 2. A true solution is a homogeneous mixture. | 2. A colloid is a heterogeneous mixture. |
| 3. A true solution is transparent. | 3. A colloid solution is translucent. |
| 4. The particles of a true solution cannot be seen even with a microscope. | 4. Some particles of colloids can be seen with a powerful microscope. |
| 5. A true solution does not scatter light. | 5. A colloid solution scatters a beam of light passing through it and renders its path visible. |
Question 5. How do the suspended particulate matter in air lead to air pollution?
Answer: Suspended particulate matter in air are a kind of air pollutant.
The various ill eff of the particulate matter present in air are the following:
(1) The particulate pollutants cause various allergic reactions. in the human body. They produce diseases like asthma, and tuberculosis, effect on eyes, etc..
(2) The particulate pollutants in air contain metal particles like lead, mercury, arsenic, zine, tin, etc. which are toxic (poisonous) to most of the living organisms; men, animals and plants.
(3) The particulate matter reduce visibility by producing haze in the atmosphere, and hinder the traffic.
(4) The particulates like smoke make our clothes and buildings dusty.
(5) The particulate suspended in air disturb the thermal balance of earth.
Question 6. How are the particulate matter containing metallic particles harmful for the human beings?
Answer: The metallic particles present in air are of lead, mercury, zinc, nickel, arsenic, etc. All the metal particles are toxic in nature. The most harmful metals for the human beings are lead and mercury. The iead particles are released into air by the exhausts of motor vehicles. Lead is a cumulative poison, it keeps on accumulating in the tissues of the human body. lt damages organs like liver, kidney and intestine and causes malformation of red blood cells in the body Asbestos dust is also a major pollutant of the atmosphere. Asbestos dust also causeslung cancer.
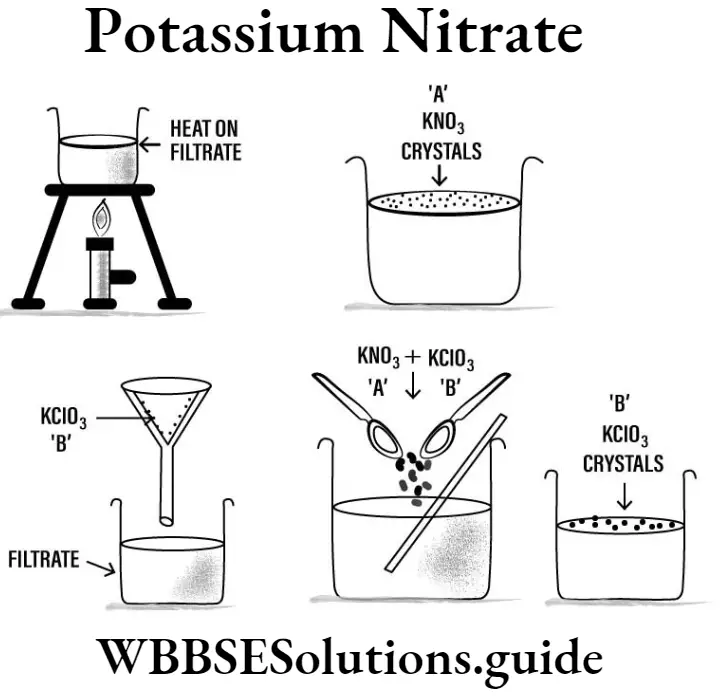
Question 7. How do you prepare the crystals of potassium nitrate in laboratory?
Answer : First prepare the saturated solution of potassium nitrate (KNO3) by dissolving potassium nitrate in water and boiling the solution for some time. After strongly heating the solution, it is allowed to cool slowly in a beaker. After some time fine crystals will separate out from the solution in a definite shape.
Concentration of solutions Class 9 WBBSE notes
Question 8. What do you mean by hydrated substances (hydrated salt) and anhydrous substances (anhydrous salt) ?
Answer :
Hydrated Substance: A substance or salt which contains certain fixed number of water molecules, bound to its one molecule, is called hydrated substance.
Anhydrous Substance: A hydrated substance which completely loses its water of crystallization is called anhydrous substance or anhydrous salt.
Examples of crystalline hydrated salts –
Copper sulphate crystals (Blue vitriol) – CuSO4. 5H2O.
Hydrated magnesium salt (Epsom Salt) – MgSO4. 7H2O
Hydrated ferrous sulphate (Green vitriol) – FeSO4 . 10H2O
Hydrated sodium carbonate (Washing soda) – NaCO4. 10H2O
Barium chloride (Barium chloride crystals) – BaCl2. 2H2O
Question 9. Give examples of some anhydrous crystalline salts.
Answer :
Some crystalline salts having no molecule of water are:
Sodium chloride – NaCl
Ammonium chloride – NH4Cl
Potassium Nitrate – KNO3
Silver nitrate – AgNO3
Potassium Permanganate – KMnO4
Potassium Dichromate – K2Cr2O7
Question 10. Show by a simple experiment that copper sulphate (blue vitriol) contains molecules of water.
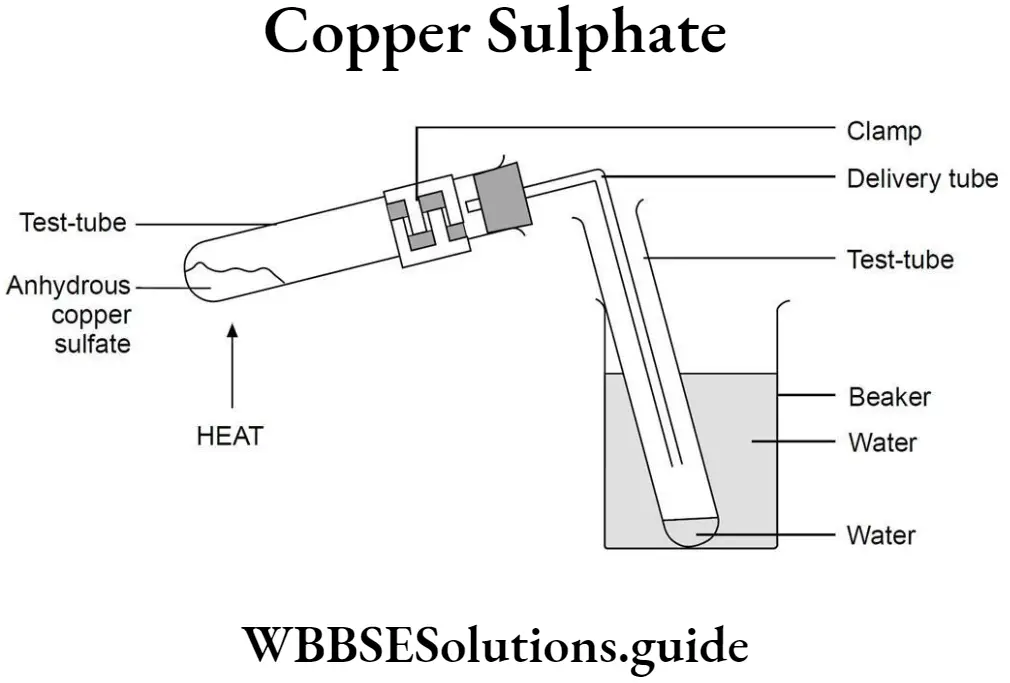
Answer :
Take some copper sulphate crystals in a hard glass test tube and strongly heat it. The crystals will slowly turn to a white powder and water drops will come out from the mouth of the test tube. Collect the water drops in a beaker. The blue crystals of copper sulphate will turn to a white powder after losing their water of crystallization.
Question 11. What are ‘Deliquescence’ and ‘Deliquescent substances’? Give an example of some deliquescent substances.
Answer :
Deliquescences: When a certain crystalline substance is placed in open air, it absorbs moisture from air and changes to liquid state, then this phenomenon is called deliquescence The crystalline substances that absorb moisture from open air and change to liquid state are called deliquescent substances.
For example Anhydrous calcium chloride (CaCl2 ),
Anhydrous sodium hydroxide (NaOH),
Anhydrous potassium hydroxide (KOH),
Anhydrous ferric chloride (FeCl3) etc.
Question 12. Describe an apparatus used to dry a substance using a drying agent.
Answer:
A simple apparatus used in laboratory to dry substances is a desiccator. A desiccator is an air tight glass vessel having a wire gauze. A drying agent Rage such as anhydrous calcium chloride is used to dry 2 wet solids.
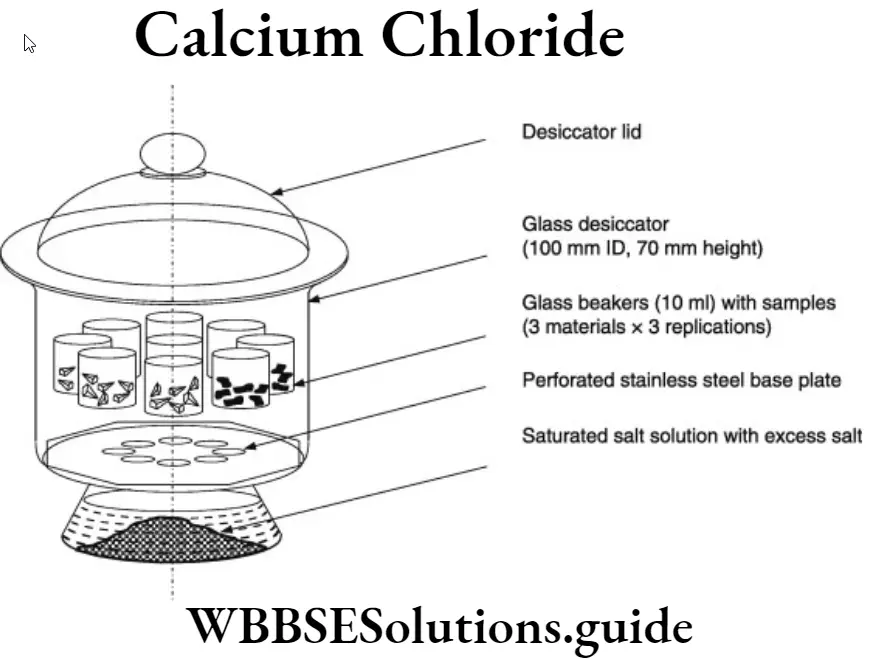
Best study material for solution WBBSE Class 9
Question 13. Discuss some different types of solutions Anhydrous calcium which do not have a liquid as a solvent.
Answer:
(1) Solution of gases in gases: According to the definition, if a gas does not interact with another, it may form a solution. Thus, a solution of oxygen (solute) in excess of nitrogen (solvent) as in air, is a solution of this type.
(2) Solution of solids in solids: Brass is a solution of a solid solute in a solid solvent. It is a homogeneous mixture of copper and zinc. Copper being present in excess is regarded as the solvent.
(3) Solution of gases in solids: The homogeneous mixture obtained by absorbing hydrogen gas in certain solid metals like palladium, nickel, etc. at a high temperature is an example of solution of gas in solids.
(4) Solution of solids in gases: The homogeneous mixture of dust particles and particles of solid fuels such as carbon, sulphur, etc. with air forms smoke and this is an example of a solution of solids in gases.
(5) Solution of liquids in gases: Minute particles of liquids suspended homogeneously in a gas medium forms mist. :
(6) Solutions of liquids in solids: Sodium-mercury amalgam, silver-mercury amalgam, etc. belong to this type of solution, where sodium and silver are the solvents and mercury is the solute.
Wb Class 9 Physical Science Question Answers
Question 14. How can you prepare a super saturated solution of a solid solute (hypo) at room temperature?
Answer:
Preparation of a supersaturated solution: Supersaturated solution can be prepared by heating a few crystals of sodium thiosulphate or hypo (Na2S2 O3, 5H2 O) in a test tube. The-crystals appear to melt or they dissolve in their own water’of crystallization and a very concentrated solution. of the salt in -water is gained. The test tube is plugged with cotton wool-and alloted to-cdol to room temperature. This solution is super saturated and even after cooling no excess soliite separates out. Ifa crystal of the salt is now added into this solution, the crystal growsup in sizé and~the whole liquid’ begins to solidify with the evolution of heat.
Question 15. Briefly describe the process of crystallisation.
Answer:
Crystallization: Crystallisation is a process from which the crystal of a substance is obtained from its solution.
Process of crystallisation for the preparation of crystal :
(1) From saturated solution of a substance: A hot saturated solution of the substance is slowly cooled and then the crystal of that substance is obtained.
(2) From unsaturated solution: An unsaturated solution is vapourised, then a concentrated solution of it is obtained. Now if this solution is gradually cooled then crystals of the substance are obtained.
(3) Sublimation process: lodine, Camphor, etc. are sublime. If the sublimate so formed is cooled then the crystals of the substances are deposited in the pot.
(4) From molten matter: Solid sulphur taken in a pot is heated to melt it and then by slowly cooling, the crystals of B-sulphur are obtained.
Question 16. Mention the uses of hygroscopic substances. Give two differences between deliquescent substances and hygroscopic substances.
Answer:
Uses of Hygroscopic substances : These substances are generally used as dying agents for many gases and solids and air. For example, ammonia gas is dried by quick- lime.
Differences between Hygroscopic and Deliquescent substances :
(1) Deliquescent substances are crystalline solids but hygroscopic substances may be non-crystalline solids or liquids.
(2) Deliquescent substances change into solution on absorbing moisture from air but hygroscopic substances do not change their states on doing so.
Question 17. State the names and uses of a few drying agents. Name one dying agent for drying
(1) Ammonia. gas
(2) Hydrogen sulphide gas
(3) Hydrogen gas
(4) Sulphur dioxide gas.
Answer:
Examples of drying agents: A few drying agents frequently used for drying other chemicals are :
(1) Anhydrous calcium chloride (CaCl2) in the desscicator in the laboratory for drying chemicals.
(2) Anhydrous sodium sulphate (Na2SO4) for drying organic solvents.
(3) Anhydrous magnesium sulphate (MgSO4) is used for drying esters, carbonyl compounds.
(4) Conc. H2SO4 is used for drying hydrogen chloride (HCI), CO2, SO2 gases.
(5) Quicklime (CaO) is used for drying ammonia gas.
(6) (1) Basic Quick lime (CaO) is used for drying basic ammonia (NH3) gas.
(2) Acidic phosphorus pentoxide (P2O5) is used for drying acidic. hydrogen sulphide gas.
(3) Cone. H2SO4 or anhydrous CaCl2 can be used for drying H, gas.
(4) Conc. H2SO4 can be used for drying SO2 gas.
Wb Class 9 Physical Science Question Answers
Question 18. (1) What do you understand by the strength of a solution?
(2) Define standard solution.
(3) How can you express the strength or concentration of a solution?
Answer:
(1) Strength of a solution: The strength of a solution means the concentration of the solute in the solution, i.e., the amount of solute present in a definite volume of the solution. The amount of dissolved solute in a definite volume of the solution determines the strength of the solution.
(2) Standard solution: A solution of known strength, i.e., a solution which contains a known amount of dissolved solute in a definite volume of the solvent is called a standard solution.
(3) Units of strength of solution: The strength or concentration of a dilute solution can be expressed by
(1) Percentage strength in weight by volume (W/V x 100)
(2) Grarn per litre (g/l).
Question 19. Point cut the main differences between suspension and colloid.
Answer:
Main differences between suspension and colloid
| Colloidal Solution | Suspension |
| 1. Size range of colloidal particles : 10A- 2000A. | 1. Particles forming suspension are of size greater than 2000A. |
| 2. Colloidal particles are invisible under powerful microscope but the scattering of light can be viewed by ultra microscope. | 2. Particles are invisible either to the naked eye or under an ordinary microscope. |
| 3. Colloidal particles readily pass through filter paper but slowly through parchment paper. | 3. Cannot pass through filter or parchment paper. |
| 4. Colloidal particles scatter light, i.e.. show Tyndal effect. | 4. Do not show Tyndal effect. |
Question 20. Describe briefly the mechanism of formation of a solution where a solid dissolves in a liquid.
Answer:
Mechanism of formation of solution of a solid in a liquid: When a solid solute comes in contact with a liquid solvent, the tiny solid particles of diameter 10-8 cm or less leave the surface of the solid solute depending on temperature and as the concentration of the solution increases, some solid particles return and deposit on the surface of the lump of solute.
At the beginning, the rate of leaving the solute surface increases and it slows down when the concentration of the solution increases. If the rate of leaving the surface of the solute is greater than that of their return, the solid dissolves totally.
Wb Class 9 Physical Science Question Answers
Question 21. Why does a lump of sugar candy dissolve more quickly in water when it is kept suspended in the water than when it is just left in the water of a tumbler?
Answer:
& jump of sugar candy if kept suspended in water, taken in a glass tumbler, dissolves more quickly than when it is dipped at the bottom of water in the tumbler. This Happens because the layers of water near the suspended lump of candy become concentrated due to dissolution of a part of the candy and become heavier and.
So move down and less concentrated and lighter water layers move near the lump. In this way, the rate of dissolution of the candy increases, i.e., it goes into solution quickly. Same thing happens if the solution is stirred, when the concentrated layers of water are displaced due to stirring. But if the lump is left at the bottom of water, the concentrated and heavier water layers remain there and water layers of less concentration hardly come near it, so the lump takes a lot of time to get dissolved.
Question 22. Does formation of a solution always accompany heat exchange?
Answer:
There is almost always heat changes accompanying formation of solutions. This means that some solutes, when go into solution, heat is evolved and for some other solutes, heat is absorbed. For example, KNO3, NH4Cl, etc. absorb heat while they go into solution, i.e., the change is endothermic, so their solubility increases with rise in temperature. In fact, solubility of most of the solid substances increases with rise in temperature, so by increasing the temperature of a solution, more solute may be dissolved in it.
Exception :
Solubility of sodium chloride (NaCl) remains almost unchanged with rise in temperature. Again, heat evolves when calcium hydroxide [Ca(OH)2], Zinc phosphate [Zn,(PO4)2] an hydrous sodium sulphate (Na2SO4), etc. go into solution, the change in such cases is exothermic, so their solubility decreases with rise in temperature. Hence, solubility of a substance of this type increases due to cooling.
Thus, in general, increase in temperature favours an endothermic change while decrease in temperature favours an exothermic change.
Wb Class 9 Physical Science Question Answers
Question 23. State the properties of a true solution.
Answer :
The main properties of a true solution are :
(1) A true solution is always clear and transparent (light can easily pass through it).
(2) A true solution is homogeneous in nature.
(3) The particles of a solute break down to almost molecule size and cannot be seen under a microscope.
(4) A true solution can easily pass through filter paper as pores of filter paper are bigger than the molecules of solution.
(5) The solute can be recovered from true solution by physical methods (as evaporating the solute or crystallization of solute).
(6) In true solution, particles of the solute do mat settle down, provided the temperatures are kept constant.
Question 24. Describe the effect of temperature and pressure on the solubility of the solids and gases.
Answer :
The effect of temperature and pressure on solubility are :
(1) The solubility of solids in liquids usually increases due to increase in temperature and decreases due to decrease in temperature.
(2) The solubility of solids in liquids remain unaffected by change in pressure.
(3) The solubility of gases in liquids usually decrease on increasing the temperature and increases on decreasing the temperature.
(4) The solubility of gases in liquids increases on increasing the pressure and decreases on decreasing the pressure.
Question 25. State with examples the effect of temperature on the solubility of different solids.
Answer:
(1) Solids whose solubility remain unaffected with rise in temperatures are :
Common salt (sodium chloride)− NaCl
Potassium chloride – KCI
Lithium chloride – LiCl
(2) Solids whose solubility increases with an increase in temperature are :
Copper Sulphate – CuSO4
Potassium Nitrate – KNO3
Sodium Nitrate – NaNO3
Ammonium chloride – NH4Cl
(3) Solids whose solubility decreases with an increase in temperature are :
Slaked line (Calcium hydroxide)
Sodium Sulphate – Na2SO4
Calcium Sulphate – CaSO4
Wbbse Class 9 Physical Science Question Answers
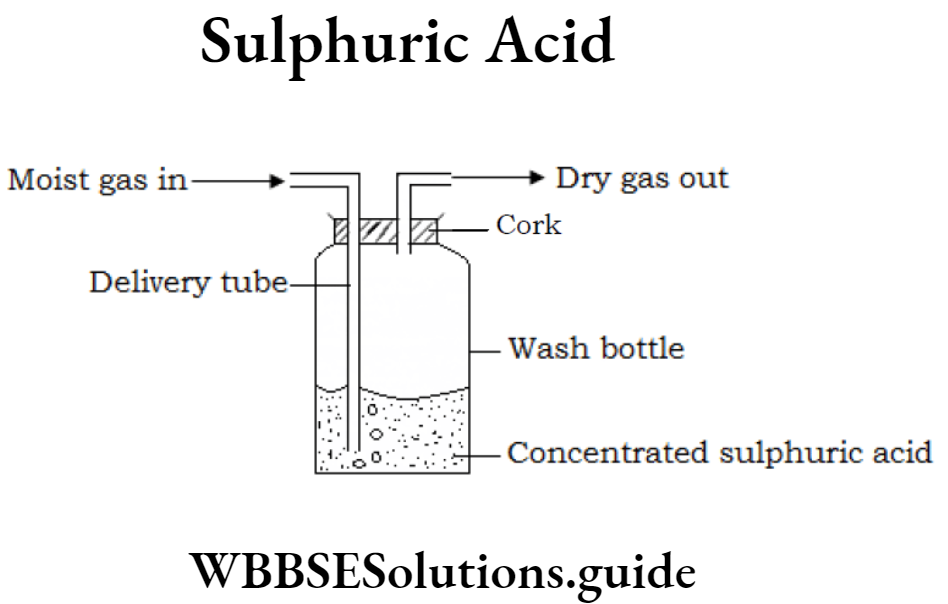
Question 26. Show by a simple experiment that concentrated sulphuric acid is used to dry a gas or air.
Answer:
Concentrated sulphuric acid is taken in a glass Moist gas Dry gas bottle fitted lightly with two glass tubes with the help of a cork. One end of a glass tube is dipped in the acid while the other glass tube end is kept above the acid level. Now a moist gas is passed through the first tube into the acid. The sulphuric acid will absorb the water vapour and the dry gas will be obtained from the second tube.
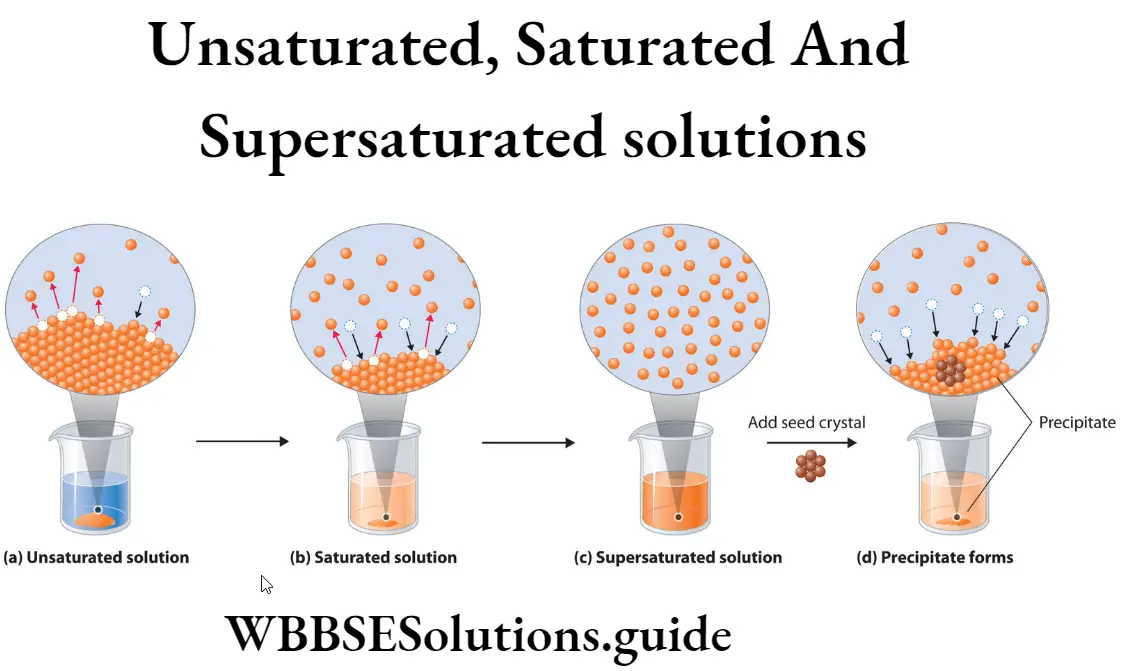
Question 27. What are unsaturated solutions, saturated solution and super-saturated solutions? Explain with examples.
Answer :
Unsaturated Solution: A solution which Drying Bottle can dissolve more of the solid solute, at a given temperature, is called unsaturated solution at that temperature.
Saturated Solution: A solution which cannot dissolve more of solute, at a given temperature, is called saturated solution at that temperature.
Super-Saturated Solution: A solution is said to be Super—Saturated when it contains in solution more of the solute than it could hold at that temperature if crystals of the solute were present.
Examples :
(1) When a teaspoon of sugar is added in a beaker containing water, it dissolves completely. The solution so formed is unsaturated.
(2) Add more sugar to the unsaturated solution of sugar in water. The solute (sugar) stops dissolving at some particular temperature. The solution is saturated at that temperature. Adding some more, sugar, it will settle down in the bottom of the beaker.
(3) On heating, the undissolved solute (sugar) will dissolve with respect to room temperature. This type of solution is called Super Saturated solution. On cooling the uper—Saturated solution, the excess. solute (sugar) appears in the form of crystals. The left solution will remain saturated.
WBBSE Class 9 Physical Science solubility and saturation solutions
Question 28. Show how you prepare 1 l of 2(M) NaCl solution.
Answer:
Calculation M \(=\frac{n}{V(\text { in I })}\)M=2\(=\frac{n}{11}\)
n=2 mol = 2 x 58.5 g NaCl = 117.0 g NaCl
So, you have to dissolve 117 g NaCl in 11 water.
Step 1: Weigh out 117 g NaCl.
Step 2: Transfer that amount of NaCl to a 1! (marked) Flask:
Step 3: Add water and fill to 1l mark.
Wb Class 9 Physical Science Question Answers
29. From the following experimental results determine the solubility of KCI at 25°C. Weight of clean and dry porcelain basin = 31.75 g.
Weight of the basin with saturated solution = 157.25 g.
Weight of the basin with dry salt = 77.25 g.
Answer: (1) Weight of the saturated KC solution = (157.25 − 31.75) = 125.5 g.
(2) Weight of KCI in the saturated solution = (77.25 − 31.75) = 45.50 g.
(3) Weight of water in saturated solution = (125.50 −45.50) = 80.00 g.
So at 25°C, 80 g water dissolves a maximum 45.50 g KCI to give a saturated solution.
∴ at 25°C 100g water dissolves \(=\frac{45.50 \times 100}{80}\)
= 56.875 g KCI to give saturated solution.
∴ Solubility of KCI at 25°C = 56.875.
Question 30. Mention the different ways to express the concentration of a solution.
Answer:
The concentration of a solution can be expressed in several different ways :
(1) Percentage by weight (% w/w): It is the amount of solute in grams present in 100 grams of the solution.
(2) Weight/volume percentage (% w/v): It is the amount of solute in grams present in 100 ml of the solution.
(3) Volume/volume percentage (% v/v): It is the volume in mi of the solute present in 100 ml of the solution.
(4) Strength: It is the number of grams of the solute dissolved per litre of the solution.
(5) Normality (N): It is the number of gram equivalent of the solute dissolved per litre of a solution.
(6) Molarity (M): It is the number of moles of solute dissolved per litre of the solution.
(7) Molality (m): It is the number of moles of solute dissloved in 1000 grams of the solvent.
(8) Formality (F): Formality of a Solution is the number of gram formula weight of the ionic solute (e.g. NaCl) dissolved per litre of the solution.
(9) Mole fraction: It is the ratio of the number of moles of one component to the total number of moles of substances in the solution.
(10) Parts per million (ppm) : It is defined as the number of parts of a component per million parts of the solution.
Question 31. Tabulate the features of true solutions, colloids and suspensions.
Answer:
| Features | True Solutions | Colloids | Suspensions |
| (1) Particles that compose the solute in solution | Atoms, ions or molecules | Groups of ions, atoms, or molecules | Large groups of insoluble particles |
| (2) Size of particles | Less than 1 mm (1 nm = 10-9 m) | 1-100 nm | Greater than 100 nm |
| (3) Separation of solute and solvent by filtering | Will not affect separation | Will not affect separation | Will affect separation |
| (4) Separation by semipermeable membrane | Will not affect separation | Will affect separation | Will affect separation |
Question 32. Differentiate between true solution, suspension and colloids.
Answer:
Differences between true solution, suspension and colloids :
| Suspension | Colloids | True Solution |
| (1) Diameter of the particle is equal to or more than 10-4cm. | The diameter of the particle is in between 10-5to l0-7 cm | Diameter of the particle is less than 10-8cm. |
| (2) Particles are visible by naked eye or by microscope. | Particles are not visible by naked eye or by microscope but can be observed by ultramicroscope. | Not visible even by ultramicroscope. |
| (3) Heterogeneous-two phase system. | Heterogeneous-two phase system | Homogeneous-one phase system. |
| (4) Cannot pass through filter paper or parchment paper. | May pass through filter paper but cannot pass through parchment paper. | Can easily pass through filter paper or parchment paper. |
| (5) The whole system looks opaque. | The who»e system looks opaque | The system is totally transparent. |
Question 33. How can saturated, unsaturated and supersaturated solutions be identified?
Answer:
Identification of saturated, unsaturated and supersaturated solutions: Excess solute must be added for identification.
(1) If on addition of the solute to a solution, the solute dissolves and the density of the solution increases, then the given solution is unsaturated.
(2) If the excess solute is left undissolved at the bottom of the container and the density of the solution decreases by the addition of a piece of solute to a solution, then the solution is super saturated.
Wbbse Class 9 Physical Science
Question 34. Write a note on different concentration units of solubility.
Answer:
Different concentration units :
(1) Percent strength: It is the number of grams of the solute present in 100 ml of the solution. Thus 10% Na2CO3 means that 10 g of Na2CO3 are present in.100 ml of the solution. 15% HNO, solution’ means 15 g of HNO3 are present in 100 ml of the solution.
(2) Gram of solute per litre : This is the number of gram of solute in 1 litre of the solution.
(3) Molarity (M) : The molarity of a solution is the number of gram moles of the solute present in one litre of the solution.
Thus if 1 mole of solute is dissolved in 1 litre of solution, the solution is said to be 1 M.
Similarly, if , \(\frac{1}{10}\)th of a mole of a solute in dissolved in 1 lit of solution, it is said to be decimolar\(\left(\frac{M}{10}\right)\)
Thus, if 1 | HC! solution contains 36.5 g HCl, the molarity is 1;
if 1 l HCI solution contains 71 g HCI, the molarity is 2.
Let a gram of solute of molecular weight m be dissolved in v lit of solution.
∴ Number of moles of solute \(=\frac{a}{m}\)
Thus v | solution contains\(\frac{a}{m}\) moles of solute.
Or, 1 | solution contains \(\frac{a}{mv}\)moles of solute.
Or, the molaritty of the solution (M)\(=\frac{a}{m v}\)=\(\frac{a / v}{m}\) .
\(\text { Or, Molarity of the solution }(\mathrm{M})=\frac{\text { Weight of the solute in gram per litre }}{\text { Gram-Molecular weight of the solute }}\) .
Wbbse Class 9 Physical Science
Question 35. write a note on the conversion of concentration of a solution from one unit to another
Answer:
Coversion of concentration of solution from one unit to another :
(1) From percent strength to molarity: If the strength of a solution be x% and the molecular weight of the solute be m, then 100 ml solution contains x of solute.
∴ 1000 ml solution contains 10x g of solute.
∴ Molarity \(=\frac{\text { Gram of solute per litre }}{\text { Gram Molecular weight }}\) (M)=\(\frac{10 x}{m} \text { (M) }\)
(2) From molarity to percent strength: If the strength of a solution is x (M), then %
Strength\(=\frac{\text { Molarity } \times \text { Gram }-\text { molecular weight of the solute }}{10}\)
(3) From molarity to gram per liter: Gram of salute per litre of the solution
= Molarity x Mol-wt.
To note that w does not depend on temperature but volume (V) depends. ae all these concentration units (which involve volume) are temperature dependent.
Question 36. What are the properties of suspension?
Answer:
Properties of suspension :
(1) Nature: Hetergeneous in nature.
(2) Visibility: Can be seen with naked eyes
(3) Size of the particle: Size of the suspended particles is nearly 10-4 cm (10-6 m).
(4) Sedimentation: Particles of suspension have a tendency to settle down when left undisturbed.
(5) Separation: Particles can be separated by filtration.
Question 37. Classify colloids with examples.
Answer:
Common Examples of Colloids :
| SI. No. | Dispersion medium | Dispersed phase | Common name of the system | Examples |
| 1 | Liquid | Gas | Foam | Soap lather, shaving cream foam, Lemonade froth, etc. |
| 2 | Solid | Gas | Solid foam | Pumice stone, foam rubber, sponge. |
| 3 | Gas | Liquid | Aerosol of liquid | Fog, mist, clouds, liquid sprays, etc. |
| 4 | Solid | Liquid | Gel | Jellies, cheese, butter, Tooth paste, etc. |
| 5 | Solid | Solid | Solid sol | Colored gemstone, colored glass, etc. |
| 6 | Liquid | Solid | Sol | Gold sol, sulphur sol, ink, paints, starch solution, muddy water, etc. |
| 7 | Gas | Solid | Aerosol of solid | Smoke, dust, etc. |
| 8 | Liquid | Liquid | Emulsion | Milk, face cream, etc. hair cream, emulsified medicine, etc. |
Question 38. What are the properties of colloids ?
Answer:
Properties of colloids :
(1) Heterogeneous nature: Colloidal solution is heterogeneous in nature having dispersed phase and dispersion medium.
(2) Visibility: Particle size being very small cannot be seen by naked eyes.
(3) Filterability: Cannot filter with ordinary filter paper, but can be separated by ultrafilters.
(4) Stability: Quite stable, the dispersed particles do not settle down on keeping for a long time.
(5) Colloidal particles show Brownian movement: The colloidal particles in dispersion medium move continuously in zig-zag path, this movement of the particles is called Brownian movement.
(6) Tyndal effect: Colloidal particles are big enough to scatter a beam of light passing through it and make its path vissible. This phenomenon is called Tyndal effect. With the help of this effect distinction between true solution and colloidal solution is made.
Question 39. Write a short note on emulsion.
Answer:
Emulsion: Emulsion is a liquid-liquid colloidal system, of which one is water and the other is an oil. Two types of emulsions are formed with two types of liquids.
(1) Oils dispersed in water (O/W type): In the system water acts as dispersion medium e.g. milk, here tiny droplets of liquid fat are dispersed in water and casein acts as an emulsifier.
(2) Water dispersed in oil (W/O type): Oil acts as dispersion medium, e.g. stiff greases, here water is dispersed in lubricating oil, butter, cod-liver oil, cold cream. Perparation of emulsion using water, oil and soap Dispersion of a liquid in the form of an emulsion is called emulsification. Emulsions are prepared by agitating a small proportion of one liquid with the bulk of the other, by passing a mixture of two liquids through a colloidal milk called homogenizer.
The emulsions prepared by shaking the two liquids are not stable and to make them stable a third substance is added in small amount during the preparation of emulsion, which is called emulsifer or emulsifying agent. The common emulsifying agents are soap, detergents, gum, gelatin, etc.
Question 40. Mention the uses of emulsifiers in daily life.
Answer:
Use of emulsifiers in everyday life : Some hydrophilic colloids are added in small quantities to the colloidal system or emulsion to stabilise and protect their colloidal nature.
Example: (1) In the commercial preparation of ice cream a little gelatin is added to protect the colloid to give a smooth taste. This little amount of gelatin prevents ice cream from forming gritty crystals of ice and lactose (sugar).
(2) Gum arabic is added to certain inks to stabilize it.
(3) The colloidal dispersion of silver bromide used in the preparation of photographic films, is stabilised by addition of gelatin.
(4) Emulsion paints are stabilised by adding the emulsifier tetrasodium pyrophosphate, sodium lauryl sulphate, etc.
Question 41. Write the effect of pressure and temperature on the solubility of gases in liquids.
Answer:
The effect of pressure and temperature on the solubility of gases in liquids (water)
(1) Effect of pressure: An increase of pressure on the surface of water causes increase in solubility of gas in water. In soda water bottle (or in cold drinks bottles} CO2 is dissolved at a higher pressure than normal pressure. Solubility of CO2 increases with increase in pressure in the solution. On opening the lid of soda water bottle, the dissolved excess gas at higher pressure rapidly bubbles out because on opening the lid the pressure on the solution suddenly decreases. There is a very little effect of pressure on the dissolution of solids.
(2) Effect of temperature: An increase in temperature of water causes decrease of solubility of gas in water. Gases are more soluble in cold water than in at higher temperature. Boiling water loses its taste because the taste of water due to the dissolved gases in it, on boiling these gases, escape out of water. Generally, solubility of solids increases with increase in temperature.
Question 42. What are the factors affecting solubility?
Answer:
Factors affecting solubility: The rate of solubility of a ‘solid in water (liquid) depends on the following factors :
(1) The particle size of the solute: Smaller is the particle size of the solute, greater is the rate of solubility. It is due to smaller particle size; greater its total surface area in contact with the solvent and greater the rate of solubility.
(2) Stirring the mixture of solute and solvent: Brings more solvent in contact with the solute and increases the rate of solution formation.
(3) Temperature: Solubility of gas in liquid decreases with rise in temperature. But the solubility of most of the solids in water usually increases with rise in temperature.
Question 43. Write a short note on solubility curves.
Answer:
Solubility curves: If the solubility of a solute in a given 10 solvent is plotted against respective temperatures, a line graph a is obtained showing the effect of temperature on the solubility of a solute. This graph is called solubility curve.
Graphical representation of temperature dependence of, solubility of following compounds in water are given. Here the solubility of substances are plotted along the ordinate (Y-axis) and the temperature along the abscissa (x-axis).
(1) Substances like KNO3 show a considerable increase in Lae solubility with increase in temperature.
(2) The solubility of NaCl increases a little with increase in temperature.
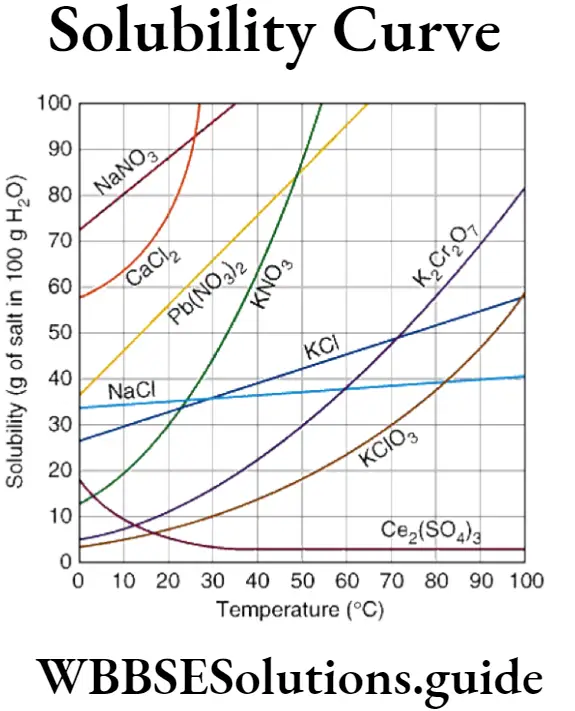
(3) Solubility of Glauber’s salt (Na2SO4,10H2O) : Its solubility rises till it reaches 32.8°C; it is the solubility of hydrated Na2SO4, 10H2O and above 32.8°C it is the solubility of anhydrous Na2SO4 salt.
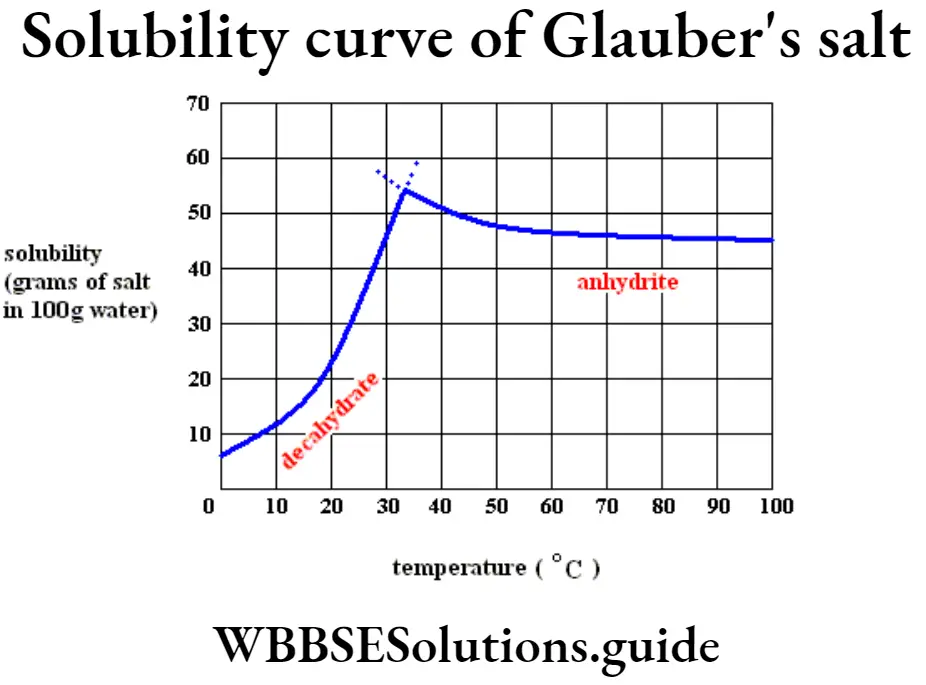 (4) The solubility curve of Ca(OH)2 shows there is a decrease of solubility after attaining certain temperature.
(4) The solubility curve of Ca(OH)2 shows there is a decrease of solubility after attaining certain temperature.
Wbbse Class 9 Physical Science
Question 44. Write about the dissolution of micromolecules and macromolecules in water.
Answer:
Dissolution of micromolecules and macromolecules in water: Macromolecules are so small that during dissolution they enter into the intermolecular space between the water molecule and get easily dissolved and form solution. But the micromolecules which are in very fine state are generally present in the form of colloid or suspension in the water.
In case of macromolecules, their size is so large that during dissolution they do not enter into the intermolecular space of water molecules, rather they displace many water molecules and go into that space and get dissolved in water. For example, bigger molecules like protein, DNA, starch, which are generally polymeric material, are always dissolved in this form.
Question 45. Write briefly various non-aqueous solvents with their uses.
Answer:
Non-aqueous solvents and their use :
| Non-aqueous solvents | Use of dissolve | Use in our everyday life |
| 1. Benzene, Toluene, Xylene chloroform | Rubber, plastics, varnishes | 1. Petrol, Kerosene, and ammonia are used in laundries for dry cleaning, for removing grease adhering on clothes. |
| 2. Turpentine od, castor oil | Paints, paraffin wax | 2. Turpentine oil is used for removing paint stain. |
| 3. Carbon disulphide | Sulphur, Phosphorus | 3. Borax solution for removing coffee and tea stain from clothes. |
| 4. Petrol | Grease, rubber, Chlorophyll | 4. Iodine solution in alcohol is called a tincture of iodine used for dressing wounds. |
| 5. Acetone | Nail Polish (Cellulose acetate) | 5. Manufacture of perfumes: Aroma substances are dissoved in alcohol. |
| 6. Alcohol | Iodine, naphthalene, chlorophyll Resin, chlorophyll. | 6. Extraction Of ChlorophyifIt is extracted by plant materials. with alcohol, petrol, etc. |
WBBSE Solutions for Class 9 Physical Science And Environment
- Chapter 1 Measurement
- Chapter 2 Force And Motion
- Chapter 3 Matter: Structure And Properties
- Chapter 4 Matter: Atomic Structure; Physical & Chemical Properties of Matter
- Chapter 5. Energy In Action: Work, Power & Energy
- Chapter 6 Heat
- Chapter 7 Sound
