Werner’s Coordination Theory
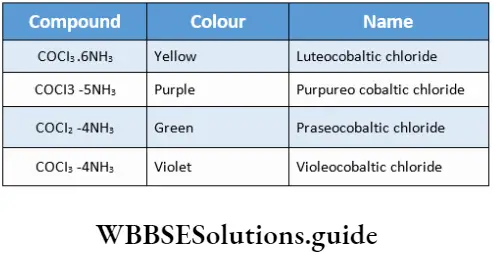
Alfred Werner, a Swiss chemist, won the Nobel Prize in 1913 for his work on the linkage of atoms and the coordination theory. Prior to him Tassaert had observed and noted that two stable compounds, cobalt(3) chloride and ammonia, combined to give a stable compound, CoCl3 -6NH3. A series of such compounds was then prepared and they were initially named on the basis of their colours.
Werner studied such compounds and, in 1893, proposed his coordination theory, which has been a guiding principle in the advancement of inorganic chemistry. Werner put forward his theory before the discovery of the electron and therefore had no idea about the electronic theory of valency.
The postulates of Werner’s theory are as follows.
- A metal exhibits two types of valency—(a) primary valency and (b) secondary valency. In modem terminology, primary valency refers to the oxidation state (number of charges on the complex ion) and secondary valency, to the coordination number. The primary valency is ionisable while the secondary valency is not.
- Every metal tends to satisfy both types of valency. The primary valency is satisfied by negative ions that neutralise the charge and the secondary valency by ligands (neutral molecules or negative ions).
- The secondary valencies are directed towards fixed positions in space.
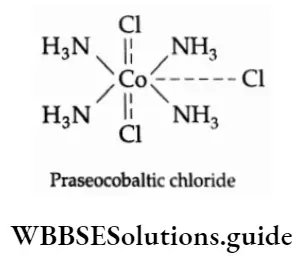
Such spatial arrangements are called coordination polyhedra. This leads to the definite geometry of coordination entities and explains isomerism. (Isomerism is noted in green and violet forms of CoCl3.4NH3.)
Werner designated CoCl3 -6NH3 as the coordination compound having three chlorines as primary valencies and six ammonia as secondary valencies.
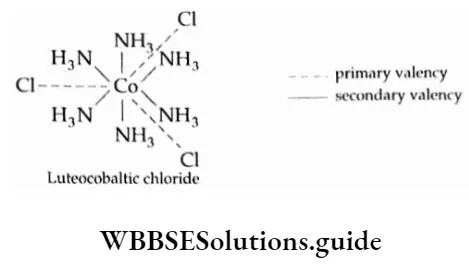
Werner formulated the complex as (CO(NH3)6]CI3. The primary valency (Werner’s theory) or oxidation state (in modern terms) of cobalt is +3, satisfied by three chloride ions. The secondary valency or coordination number is 6, satisfied by six ammonia molecules.
The coordination sphere comprises cobalt and six NH–, ligands which are nonionisable. The ionisation sphere comprises three ionisable Cl ions. The complex dissociates to give four ions, [CO(NH3)6]3+ and 3Cr.
When one mole of the compound is treated with excess silver nitrate, three moles of AgCl are precipitated. CoCl3 -5NH3 is formed from CoCl3•6NH3 by the loss of one ammonia molecule.
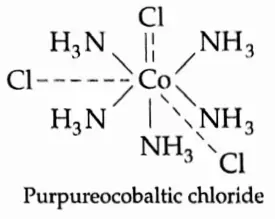
The complex was formulated as [Co(NH3)5CI]CI2. Werner did this in accordance with postulate 2, which states that both primary and secondary valencies must be satisfied. There are five NH3 molecules, so one Cl– serves the dual purpose of satisfying primary and secondary valencies.
Such a Cl– is nonionisable. On adding silver nitrate two C–I ions are precipitated as AgCl and the complex ionises to give [Co[NH3)5Cl]2++ 2CI–.
Extending the theory to CoCl3.4NH3, Werner gave the following formula for the compound: [CO(NH3)4Cl2 ]+ Cl. This gives 1 mole of AgCl with silver nitrate.
Postulate 3 of the theory deals with the stereochemistry of coordination entities. Werner suggested that six-coordinate complexes have octahedral structures whereas four-coordinate complexes have square planar or tetrahedral structures.
Example What are the coordination entities and counter ions in the given compounds? How do they ionise in solution?
⇒ \(\left[\mathrm{Cr}\left(\mathrm{NH}_3\right)_6\right] \mathrm{Cl}_3,\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_3 \mathrm{Cl}_3 \mathrm{l}, \mathrm{K}_4\left[\mathrm{Fe}(\mathrm{CN})_6 \mathrm{l}, \mathrm{Na}_2\left[\mathrm{PtCl}_4 \mathrm{l}, \mathrm{Ni}(\mathrm{CO})_4, \mathrm{~K}_2\left[\mathrm{Ni}(\mathrm{CN})_4\right]\right.\right.\right.\)
Solution table
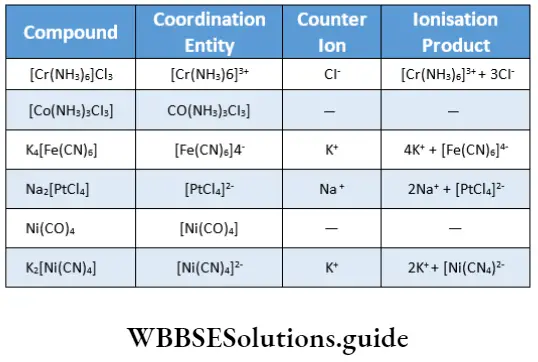
[CO(NH3)3CI3]and Ni(CO)4 do not have counter ions.
Example Specify the oxidation numbers of metals in the following.
- [Cr(CN)(H2O)(en)2]2+
- [PdBr4]2-
- [CoCI3(H2O)3]
- [CrCI2(en)2]+
- K3(Cr(CN6)]
Solution
To find the oxidation numbers of the respective metals in each case, let us assume the oxidation number to be x
(1) Oxidation state of CN = -1 and H2O and en are neutral.
∴ x-1 +0 + 0 = 2, ∴ x = +3.
(2) The oxidation state of Br = -1.
∴ x + 4(-l) = -2
or x-4 = -2, ∴ x = +2.
(3) The oxidation state of Cl = -1 and H2O is neutral.
x + 3{-1) + 0 = 0 or x-3 = 0, ∴ x = +3.
(4) Oxidation state of Cl = -1 and en is neutral.
x + 2(-1) + 0 =1 or x- 2 =1, ∴ x = +3.
(5) The oxidation state of K = +1 and
oxidation state of CN = -1.
∴ 3×1+ x + 6(-1) = 0
or 3 + x- 6 = 0 or x- 3 = 0, .’. x = +3
Example Write the correct formula for the following coordination compounds:
- CrCl3•6H2O (violet with 3 chloride ionslunit formula)
- CrCl3• 6H2O (light green with 2cI–/unit formula)
- CrCl3 6H2O (dark green with1cI–/unit formula)
Solution
- Since there are 3CI–/unit formula, the primary valency of cobalt is 3 and the 3CI– ions are in the ionisation sphere.
Therefore, the formula is [Co(H2O)6]Cl3. - There are 2cI– in the ionisation sphere therefore formula is [Co(H2O)5CI]CI2•H2O.
- There is only one chloride ion in the ionisation sphere. Therefore, the formula is [Co(H2O)4Cl2]CI•H2O.
Nomenclature Of Coordination Compounds
A comprehensive system of nomenclature of coordination compounds has been given by the IUPAC. The basic rules for the systematic naming of coordination compounds are summarised as follows.
Rules For Naming A Mononuclear Complex
1. Order of listing ions The cation is named first followed by the anion. This is the normal practice while naming salt. For example,
(1) NaCl—sodium chloride
(2)[CO(NH3)6]CI3—hexaamminecobalt(HI) chloride
Here the cation is [Co(NH3)6]3+
(3) Nonionic complexes are given a one-word name
[PtCl2(NH3)2]—diamminedichoroplatinum(2)
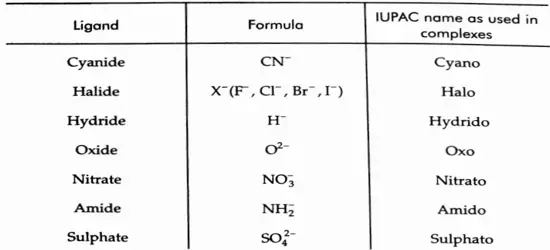
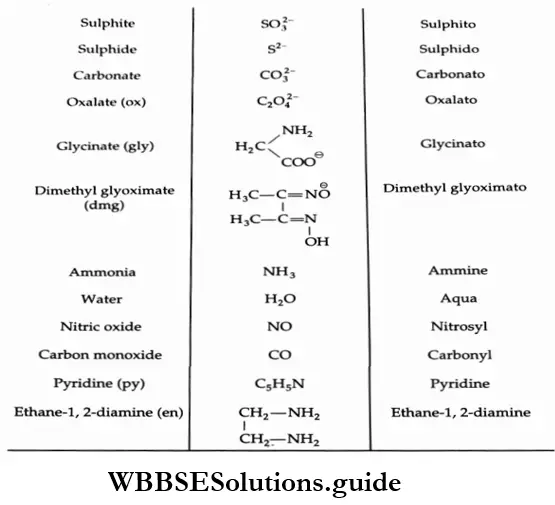
2. Names of ligands While naming, the negative ligands end in -O, for example, chloro (Cl– ) and cyano (CN–)Neutral ligands have no special ending for example, aqua (H2O), ammine (NH3) and carbonyl (CO).
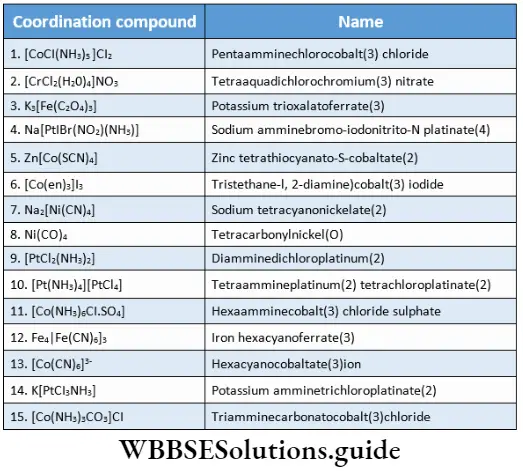
3. Order of listing ligands Ligands are named alphabetically prior to [CoBrCl(NH3)4]CI is called tetraammine bromochlorocobalt (3) chloride.
4. Numerical prefixes The prefixes di-, tri-, tetra-, penta-, etc., indicate the number of ligands of the same type. When the name of the ligand already includes such a prefix (like that in ethane-1, 2-diamine) t en to avoid confusion the modified prefixes bis-, tris-, tetrakis-, pentakis- are used and the ligand is p ce in parentheses.
For example, [Co(en)2]2(SO4)3 is named as bis(ethane-l, 2-diamine)cobalt(3) sulphate, ere en is NH2CH2CH2NH2.
5. Termination of names Cationic and neutral complexes have no special ending whereas anionic complexes terminate in -ate. example [Ag(NH3)2]+ is diamminesilver(1) ion and K2[PtCl6] is named potassium hexachloroplatinate(II). Sometimes Latin names of metals are used in anionic complexes.
The following examples illustrate this:
K3[Fe(CN)6] Potassium hexacyanoferrate(3)
Na2 [SnCl4] Sodium tetrachlorostannate(2)
K[AuCl4] Potassium tetrachloroaurate(1)
6. Oxidation number The oxidation number of the central metal atom is indicated in Roman numerals in parentheses after the name of the metal. In the examples given below, the oxidation number of cobalt is 3.
[CoCl2(NH3)4]NO3 Tetraamminedichlorocobalt(3) nitrate.
7. In the case of an ambidentate ligand, the atom coordinated to the central atom is indicated by writing the symbol of the donor atom in bold letters after the name of the ligand separated by a hyphen.
The following examples illustrate the rule.
Na3[Cr(SCN)6] Sodium hexathiocyanato-S-chromate(3)
Na3[Cr(NCS)6] Sodium hexathiocyanato-N-chromate(3)
The ambidentate nitrite ion may donate through the nitrogen or the oxygen atom. It is then referred to as nitrito-N and nitrito-O accordingly.
The names of some common ligands are given in followed by some examples of coordination compounds and their names
Rules For Formulating A Mononuclear Complex
1. Order of listing
- The central atom is listed first.
- The ligands are then listed alphabetically. The negative ligands are listed before the ligands. Polydentate ligands are also listed alphabetically.
2. Use of brackets The formula of the coordination entity is enclosed in square brackets, atomic and enclosed in parentheses.
3. Balancing of charges The charge of the cation(s) is balanced by the charge of the anion(s).
The following examples illustrate the rules.
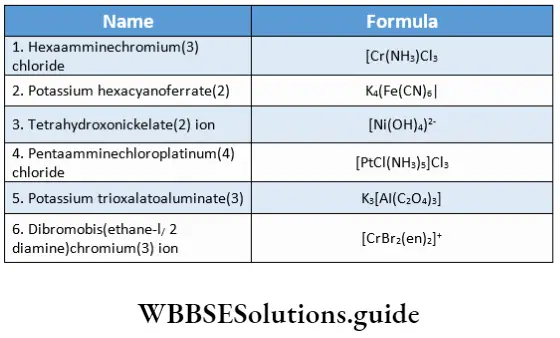
Example Using IUPAC norms write the names of the following coordination entities and compounds.
- [CoCI(NO2)(NH3)4]CI
- [CuCI2(CH3NH2)2]
- [PtCl(NH2CH3)(NH3)2]Cl
- [Mn(H2O)6]2+
- [Co(en)3]3+
- [Cd(SCN)4]2+
- [Ti(H2O)6]3+
Solution
- Tetraamminechloronitrito-N-cobalt(3) chloride
- Dichlorobis(methylamine)copper(2)
- Wamminechloromethylamine platinum(2) chloride
- Hexaaquamaganese(2) ion
- Tris(ethane-1, 2-diamine)cobalt(3) ion
- Tetrathiocyanato-S-cadmium(2) ion
- Hexaaquatitanium(3) ion.
Example Using IUPAC norms write the formulae of the following.
- Tetrahydroxozincate(2) ion
- Hexaamminecobalt (3) sulphate
- Potassium tetrachloropalladated
- Potassium trioxalatochromated(3)
- Diamminedichloroplatinum(2)
- Hexaammineplatinum(4)
- Potassium tetracyanonickelate(2)
- Tetrabromocuprate(2)
- Pentaamminenitrito-O-cobaltate(3)
- Pentaamminenitrito-N-cobaltate(3)
Solution
- [Zn(OH)4]2-
- [Co(NH3)6]2(SO4)3
- K2[PdCl4]
- K3[Cr(C2O4)3]
- [PtCl2(NH3)2]
- [Pt(NH3)6]4+
- K2[Ni(CN)4]
- [CuBr4]2-
- [Co(ONO)(NH3)5]2-
- [Co(NO2)(NH3)5]2-
Isomerism In Coordination Compounds
Two or more compounds having the same molecular formula but different structural formulae and therefore different properties are known as isomers.
Coordination compounds exhibit two main types of isomerism – Structural isomerism and stereoisomerism.
These may be further classified as follows.
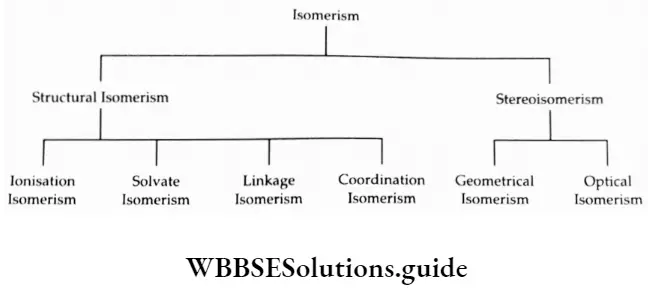
Ionisation Isomerism
Compounds having the same composition but yielding different ions in solution are called ionisation isomers. The isomerism arises due to the interchange of groups within and outside the coordination spheres. This happens when the counter ion in a complex salt is itself a potential ligand.
There are two isomers corresponding to the formula Co(NH3)5 BrSO4. One is red-violet yielding a white precipitate with BaCl2 and is represented as [CoBr(NH3)5]SO4. The other is red and gives a pale yellow precipitate with AgNO3 and is represented as [Co(SO4)(NH3)5]Br.
Other examples of the pairs of compounds which produce different ions in solution are [PtCl2(NH3)4 |Br2 and [PtBr2 and (NH3)4]CI2; [Co(NO3)(NH3)]NO3
Solvate Or Hydrate Isomerism
Hydrate isomers have the same molecular formula but differ in the number of water molecules present as ligands (in the coordination sphere) or as molecules of hydration (outside the coordination sphere).
There are three distinct hydrate isomers of the compounds with the molecular formula CrCl3 -6H2O. They differ in colour and properties.
The three isomers can also be identified by the addition of excess silver nitrate to their aqueous solutions and on heating with concentrated sulphuric acid as given below.

Other examples of hydrate isomers are as follows.
[CoCl(H2O)(en)2]CI2 and [CoCl2(en)2 ]CI•H2O;
[CoCI(H2O)(NH3)4 ]Br2 and [CoBrCl(H2O)(NH3)4 ]Br•H2O
Linkage Isomerism
Linkage isomerism occurs in coordination compounds containing ambidentate ligands. The isomers differ in the mode of attachment of the ambidentate ligand.
For example, the NO2 ion can be attached either through the nitrogen or the oxygen atom and the SCN– ion may be attached through either the sulphur or the nitrogen atom. The compound [Co(NO2)(NH3)5]CI2 is yellow and here the nitrogen atom of NO2– acts as the electron pair donor.
The other isomer [Co(ONO)(NH3)5]CI2 is red and a nitrite complex—it contains the Co(ONO) link. Na2 [Pt(SCN)4] and Na2 [Pt(NCS)4] form another example of linkage isomerism.
Coordination Isomerism
This type of isomerism is exhibited when both the cation and anion are complex ions and the interchange of ligands between cationic and anionic entities of different metal ions takes place. The isomers differ in the distribution of ligands in the cation and anion.
Some examples are as follows.
- [Co(NH3)6][Cr(CN)6] and [Cr(NH3) 6][Co(CN)6]
- [Cu(NH3)4][PbCl4] and [Pt(NH3) 4][CuCl4]
- [Co(en)3][CrCl6] and [Cr(en)3][CoCl3]
Geometric Isomerism
This type of isomerism arises due to ligands occupying different positions around the central metal ion. The ligands in question may be adjacent to each other (cis form) or opposite to each other (transform).
Hence this is also referred to as cis-trans isomerism. It is most common in square-planar and octahedral complexes.
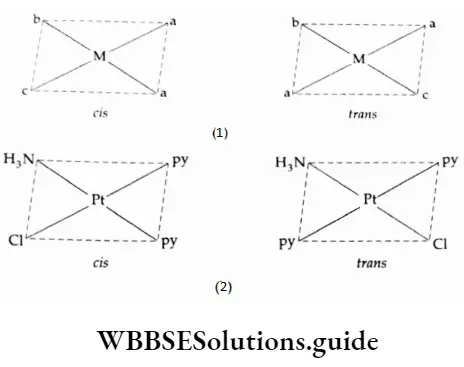
1. Square-planar complexes
Square-planar complexes of the type [Ma2b2] and [Ma2bc] (where M is the metal and a, b, c are monodentate ligands) show geometrical isomerism.
The examples shown illustrate the point.
![Coordination Compounds and Organometallics The cis and trans isomers of the square-planar [Ma2b2] type and The cis and trans isomers](https://wbbsesolutions.guide/wp-content/uploads/2023/10/Coordination-Compounds-and-Organometallics-The-cis-and-trans-isomers-of-the-square-planar-Ma2b2-type-and-The-cis-and-trans-isomers.png)
Apart from colour, the isomers differ in physical and chemical properties. The cis isomer has a finite depth moment whereas the trans isomer has zero dipole moment, cis [PtCl2(NH3)2] is referred to as cis-platin and used in the treatment of cancer.
Square-planar complexes having unsymmetrical bidentate ligands or ligands whose donor atoms are different, for example, glycine where one donor atom is N and the other is O, exhibit this isomerism.
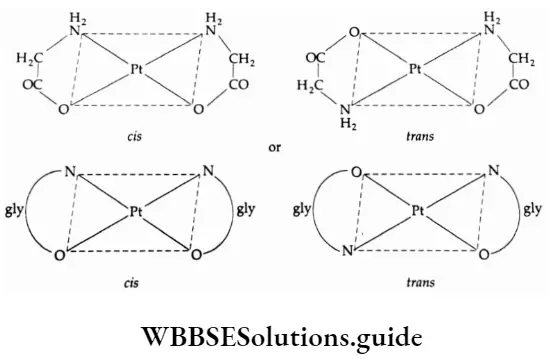
It may be noted that square-planar complexes of the type Ma3b and Mab3 do not show geometrical isomerism as in all cases the spatial arrangement around the central metal is the same. Tetrahedral complexes also do not show geometrical isomerism because, in a tetrahedral geometry, all positions are adjacent to each other.
2. Octahedral complexes
Octahedral complexes of the type (Ma4b2 ], [Ma2b4 ], [Ma3b3 ] and [Ma4be] exhibit geometric isomerism. Let us consider the six positions of an octahedron.
The trans-positions are 1-6, 2-4 and 3-5 while the cis-positions are 1-2, 1-3, 1-4, 1-5, 2-3, 6-3, 6-4, etc.
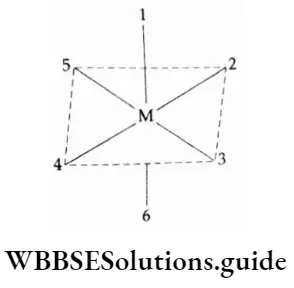
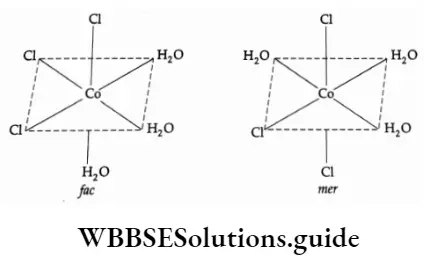
Geometrical isomerism in octahedral complexes of Ma3b3 types can also be of facial (fac) or meridional (mer) type.
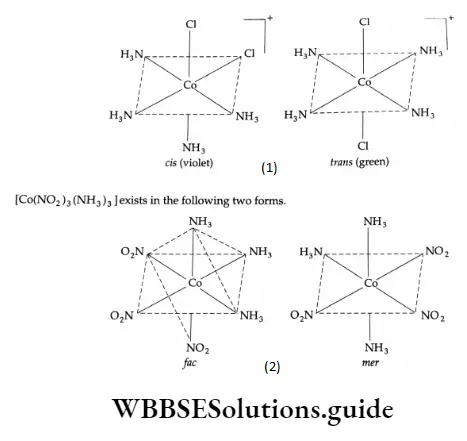
In the face form a set of similar ligands forms one face of the octahedron and the isomer is called the facial (fac) isomer. On the other hand, when the positions are around the meridian of the octahedron, we get the meridional isomer (mer).
Optical Isomerism
Optical isomers or enantiomers are pairs of molecules which rotate a plane of polarised light equally but in opposite directions. The isomers are non-superimposable mirror images of each other and have no plane of symmetry.
The only property that distinguishes the two isomers is the rotation of the plane of polarised light in a polarimeter. The isomer is dextrorotatory (d) or (+) if it rotates the plane of the polarised light to the right and laevorotatory (1) or (-) if the plane of the polarised light is rotated to the left. A mixture containing equal amounts of d and l or isomers is a racemic mixture with a net zero rotation.
Optical isomerism is common in octahedral complexes having bidentate ligands. Square planar complexes are seldom optically active.
Some typical examples of optically active octahedral complexes are as follows.
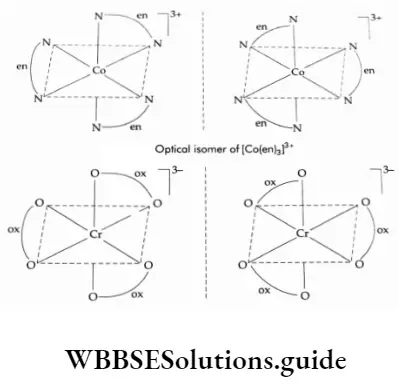
1. In a coordination entity of the type [M(AA)3 ], where AA is a symmetrical bidentate ligand the complex exists in two forms.
For example, in [Co(en)3]3+ and [Cr(ox)3]5- the symmetrical bidentate ligands are ethane-1, 2-diamine (en, N donor) and the oxalate ion (ox, O donor) respectively. Here en represents H2 NCH2 CH2 NH2.
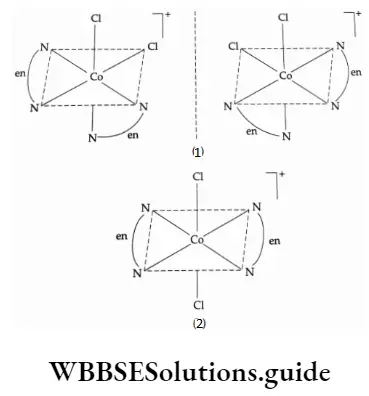
In the case of M(AA)2a2] and [M(AA)2ab] type of coordination entities where AA is a symmetrical, bidentate ligand and a, b are monodentate ligands only the cis-isomer shows optical activity.
For example, [CoCl2(en)2 ]+ exhibits geometrical isomerism and only the cis form is optically active. The transform, which is superimposable on its mirror image, is optically inactive.
3. |M(AA)b2c2 ] type complexes exhibit optical activity, example [CrCl2(NH3)2(en)]+
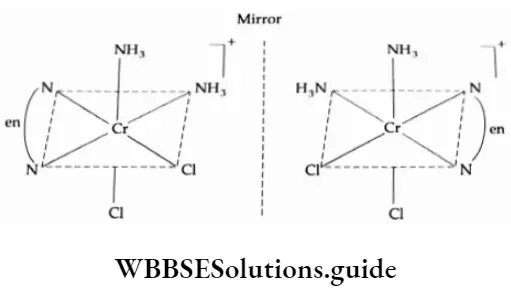
As you can see, the structure is not superimposable in its mirror image.
Example Predict the type of isomerism in the following compounds.
- [PtBr(NH3)3 INO2 and [PtNO2(NH3)3]Br
- [Cr(ox)3]3-
- [CoCl2(en)2]+
Solution
- Ionisation isomerism
- Optical isomerism
- Geometrical isomerism; cis form will be optically active.
Example How will you distinguish between [Co(NH3)5SO4]Br and[Co(NH3)5Br]SO4?
The complex [Co(NH3)5SO4 ]Br in solution ionises to give [Co(NH3 )5SO4]+ and Br–. Therefore, on adding AgNO3 to the solution, a pale yellow precipitate of AgBr is obtained.
On the other hand, the complex [CO(NH3)5 Br]SO4 will yield [Co(NH3)5Br]+ and SO4– in solution. On adding BaCl2 to the solution, a white precipitate of BaSO4 is obtained.
Solution
Example How many geometric isomers are possible in the following?
- [(Co(en)3]3+
- [CrCl3[H2O)3]
Solution
- None
- Two
Bonding In Coordination Compounds
Werner was the first to make an attempt to describe the bonding in coordination compounds. He did not have at his disposal any of the modem instruments and techniques to deduce the structure of coordination complexes.
The Werner theory put forward in 1893 was not based on any theoretical principles. Moreover, the electron was discovered later in 1896 by Sir Thomson.
Once the importance of the electron in chemical bonding was established, Sidgwick and Lowry suggested that the primary and secondary valencies of Werner’s theory actually ionic and covalent (coordinate) bonds respectively. Werner’s theory could not explain
- why do only transition metals form coordination compounds
- the directional nature of the bonds
- characteristic magnetic and optical properties of coordination compounds.
Many theories, that too after 1930, were extended to coordination compounds in order to explain the above points. These are Valence Bond Theory (VBT), Crystal Field Theory (CFT), Ligand Field Theory (LFT) and Molecular Orbital Theory (MOT). At this level of learning, we will restrict ourselves to an elementary treatment of the application of VBT and CFT to coordination compounds.
Metal Carbonyls
Metal carbonyls are a class of compounds involving carbon monoxide (CO) as a ligand. The first carbonyl to be synthesised was tetra carbonyl nickel Ni(CO)4. Carbonyls may be homoleptic or heteroleptic. In homoleptic carbonyls, the metal is bonded only to carbonyl ligands whereas in heteroleptic carbonyls, the metal is attached to other ligands, in addition to carbonyl.
Most of the transition metals form stable homoleptic carbonyls. For example, Cr(CO)6, Mo(CO)6, W(CO)6, Mn2(CO)10, Fe(CO)5 , Fe2(CO)9, Fe3(CO)12, Co2(CO)8, Ni(CO)4, etc. The structures of some metal carbonyls are shown below.
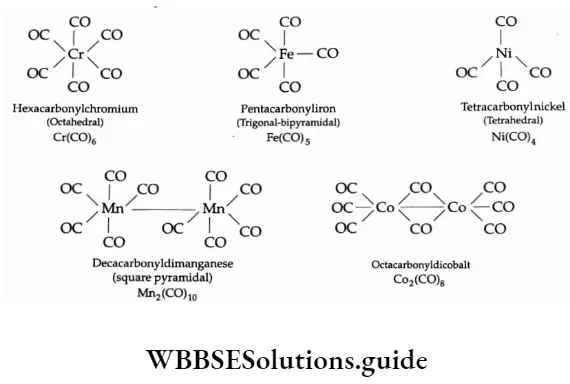
The mononuclear carbonyls (involving one metal atom) have simple structures like octahedral, trigonal bipyramidal, tetrahedral, etc. In Mn2(CO)10, two square pyramidal Mn(CO)5 units are joined by an Mn—Mn bond. In Co2(CO)8 there is a Co—Co bond and two carbonyl groups act as a bridge between the metal atoms.
The mononuclear carbonyls are volatile and toxic. They are colourless or light-coloured at room temperature and pressure. The iron and nickel mononuclear carbonyls are exceptions—they are liquids.
Carbonyls are soluble in hydrocarbon solvents, with the exception of Fe2(CO)9. Polynuclear carbonyls are deeply coloured, example Fe3 (CO)12, dodecocarbonyltriiron is a dark grass green solid.
Bonding in carbonyls
For convenience, the study of bonding in metal carbonyls may be considered to be a two-step process. In the first step, a weak cr-bond is formed by the donation of electrons from the carbonyl ligand to a vacant hybrid orbital of the metal.
In this process, carbon monoxide acts as a weak electron pair donor, i.e., a Lewis base. The weak cr-bond is strengthened by rt-bonding, which is the second step. In this step, the filled d orbitals of the transition metal overlap with a vacant antibonding (π) molecular orbital of carbon monoxide.
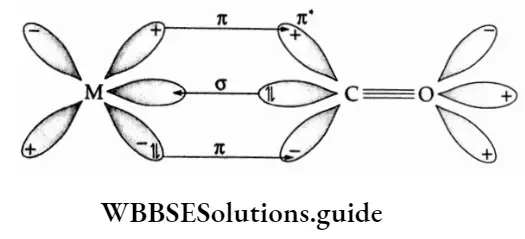
Thus CO is also an electron pair acceptor, i.e., a Lewis acid. Therefore carbon monoxide is referred to as an o-b.ise and jo-add because during o-bond formation it acts as a Lewis base and during π-bond formation, as a Lewis acid. Such bonding is referred to as synergic bonding.
Applications Of Coordination Compounds And Organometallics
Coordination Compounds
Coordination compounds find manifold applications in biology, analytical chemistry and industry. Some of them are discussed as follows.
Biological systems
You have already studied chlorophyll (green photosynthetic pigment) and haemoglobin (red pigment of blood which transports oxygen), the two vital compounds in biological systems.
They are coordination compounds containing the macrocyclic porphyrin ligand attached to magnesium and iron respectively.
Myoglobin, the reservoir of oxygen in higher animals, and vitamin B12 are also coordination compounds of iron and cobalt. Many enzymes like carbonic anhydrase and carboxypeptidase A are also coordination compounds.
Analytical Chemistry
Coordination compounds are used in qualitative and quantitative analysis. The colour reactions undergone by metal ions with the different chelating ligands are highly sensitive and specific. They are used for the detection of micro amounts of metal ions.
For example, the cherry-red colouration of nickel(II) with dimethylglyoxime and the Prussian blue colouration of ferric ions with potassium ferrocyanide is a result of complex formation.
Ethylenediaminetetraacetic acid (EDTA) is a versatile chelating agent and is used in complexometric titrations (complexometric titration is a type of titration based on complex formation between the analyte and titrant) to estimate various metals.
A common application of EDTA titration is to determine the hardness of water by estimating the amount of Ca2+ and Mg2+ ions present in the sample.
Metallurgy
Complex formation is also used in the extraction processes of the noble metals silver and gold. When extracted from their ores silver and gold combine with potassium cyanide to form the complexes [Ag(CN2]– and [Au(CN)2]– respectively in an aqueous solution. The metals are then precipitated from this solution by adding a more electropositive metal zinc, which displaces the noble metal from the complex.
Industry
- Coordination compounds are used as catalysts in many chemical reactions. For example, [Co(CN)5]3- is used for the hydrogenation of alkenes. Tungsten and molybdenum complexes are used for chemical nitrogen fixation.
- The cyanide complexes of silver and gold are used for silver and gold plating of metal articles.
- Sodium thiosulphate (hypo solution) is used as a fixer in photography. It dissolves the undecomposed silver halides as a soluble coordination compound. The complex ion formed is [Ag(S2O3)2 ]3“.
- Many common dyes and pigments are coordination entities, for example, phthalocyanin blue.
- Many chelating agents are used as antidotes in cases of metal poisoning. EDTA is used in the treatment of lead poisoning. D-penicillamine and desferrioxime B are chelating ligands used to remove excess copper and iron. Cis diamminedichloroplatinum(2), known as cis-platin, is used as an anti-cancer agent.
Organometallics
- Organometallic compounds are widely used as catalysts in industrial processes. They may act as homogeneous (soluble in the reaction medium) or heterogeneous (insoluble in the reaction medium) catalysts.
Examples of two important catalysts are chlorotic (triphenylphosphine)rhodium(1) (Wilkinson’s catalyst) used in the hydrogenation of alkenes and triethylaluminium which along with titanium tetrachloride (Ziegler Natta catalyst) is used in low-temperature polymerisation of alkenes. - Organolithium and organomagnesium compounds are used for the synthesis of many organic compounds.
- Organoarsenic compounds are used in the treatment of syphilis. New drugs are synthesised nowadays by using metallocenes.
- Ethylmercury chloride is used in agriculture to prevent infection in young plants.
Coordination Compounds And Organometallics Multiple-Choice Questions
Question 1. Potassium ferricyanide is a
- Double Salt
- Mixed Salt
- Chelate
- Complex
Answer: 4. Complex
Question 2. EDTA is a
- Hexadentate Ligand
- Monodentate Ligand
- Bidentate Ligand
- Tridentate Ligand
Answer: 1. Hexadentate Ligand
Question 3. Which reagent is used to identify nickel?
- 2, 2′-Dipyridyl
- Dimethylglyoxime
- Potassium Ferrocyanide
- Potassium Ferricyanide
Answer: 2. Dimethylglyoxime
Question 4. The IUPAC name for [CoCl3(NH3)2(H20)] is
- Diammineaquatrichlorocobalt(3)
- Aquatrichlorodiamminechlorocobalt(3)
- Diammineaquatrichlorocobaltate(3)
- Diamminetrichloroaquacobalt(3)
Answer: 1. Diammineaquatrichlorocobalt(3)
Question 5. In K4[Fe(CN)6] the hybridisation of the central metal is
- sp3
- dsp2
- d2sp3
- sp2
Answer: 3. d2sp3
Question 6. A complex involving square planar geometry displays the hybridisation
- sp3
- d2sp
- sp3d
- dsp2
Answer: 4. dsp2
Question 7. SCN– is an example of an
- Ambidentate Ligand
- Bidentate Ligand
- Tridentate Ligand
- Chelating Ligand
Answer: 1. Ambidentate Ligand
Question 8. The oxidation number of Co in [Co(en)3]2(SO4)3 is
- +2
- +3
- +4
- +5
Answer: 2. +3
Question 9. Which among the following is colourless?
- [Ti(H2O)6]3+
- [Ti(NO3)4]
- [Cr(NH3)6]3+
- [Ni(H2O)6]2+
Answer: 2. [Ti(NO3)4]
Question 10. [Co(NH3)5NO3]SO4 and [Co(NH3)5SO4]NO3 exhibit
- Coordination isomerism
- Linkage isomerism
- Optical isomerism
- Ionisation isomerism
Answer: 4. Ionisation isomerism
Question 11. What is the coordination number of the metal in [Mn(en)2Cl2]?
- 6
- 4
- 5
- 3
Answer: 1. 6
Question 12. What is the oxidation state of iron in K4[Fe(CN)6]?
- 1
- 2
- 3
- 4
Answer: 2. 2
Question 13. Which of these is correct?
- CuSO4 -5H2O is colourless.
- Fe(CO)5 is an organometallic compound.
- Zn2+ forms coloured compounds.
- [Ni(CN)4]2– is tetrahedral.
Answer: 2. Fe(CO)5 is an organometallic compound.
Question 14 How many ions are formed when [Co(NH3)6]CI3 is dissolved in water?
- 6
- 3
- 4
- 2
Answer: 3. 4
Question 15. Which of these ligands forms a chelate?
- Acetate
- Oxalate
- Ammonia
- Cyanide
Answer: 2. Oxalate
Question 16. The structure of Fe(CO)5 is
- Tetrahedral
- Square Pyramidal
- Octahedral
- Trigonal Bipyramidal
Answer: 4. Trigonal Bipyramidal
Question 17. Which among the following is the most stable?
- [Co(NH3)6]3+
- [CoF6]3–
- [CoCl6]3–
- [CoI6]3–
Answer: 1. [Co(NH3)6]3+
Question 18. Which among the following will not exhibit geometrical isomerism?
- [Cr(NH3)4Cl2]+
- [Pt(NH3)2Cl2]
- [Cr(NH3)3Cl3]
- [Cr(NH3)5Cl]2+
Answer: 4. [Cr(NH3)5Cl]2+
Question 19. Which of these will show both geometrical and optical isomerism?
- [Co(en)2CI2]+
- [Co(NH3)5CI]2+
- [Co(NH3)4Cl2]+
- [Co(OX)3]3–
Answer: 1. [Co(en)2CI2]+
Question 20. The IUPAC name for [Co(NH3)6][Cr(CN)6] is
- Hexaamminecobalt(3) Hexacyanochromate(3)
- Hexaamminecobaltate(3) Hexacyanochromium(3)
- Hexaamminecobalt(3) Hexacyanochromium(3)
- Hexaamminecobaltate(3) Hexacyanochromate(3)
Answer: 1. Hexaamminecobalt(3) Hexacyanochromate(3)
Question 21. The oxidation number of Pt in K[Pt(C2H4)Cl3] is
- 4
- 2
- 3
- l
Answer: 2. 2
Question 22. The Ziegler-Natta catalyst contains
- Iron
- Rhodium
- Titanium
- Magnesium
Answer: 3. Titanium
Question 23. The oxidation number of Ni in [Ni(CO)4] is
- 0
- 1
- 2
- 3
Answer: 1. 0
Question 24. Which will yield Fe3+ in solution?
- [Fe(CN)6]3-
- Fe(CN)6]4–
- FeSO4
- K2SO4 .Fe2(SO4)3
Answer: 4. K2SO4 .Fe2(SO4)3

