Osmosis
Osmosis Definition: The physical and passive process by which the movement of solvent or water molecules between two solutions of different concentrations, occurs from the region of their higher concentration (lower DPD) to lower concentration (higher DPD), across a semipermeable membrane, is called osmosis.
Osmosis Characteristic features:
- Osmosis is a special type of diffusion between two similar solutions with different concentrations. It may also involve diffusion between a solution and its pure solvent.
- No expenditure of metabolic energy takes place during this physical process.
- During osmosis, solvent molecules spontaneously move from a region of its higher concentration to a lower concentration, through a semipermeable membrane. The movement continues until the concentration of both solutions becomes equal.
- The membrane that is used in osmosis is perfectly semipermeable, which means that it is freely permeable to solvent molecules but impermeable to all solute molecules.
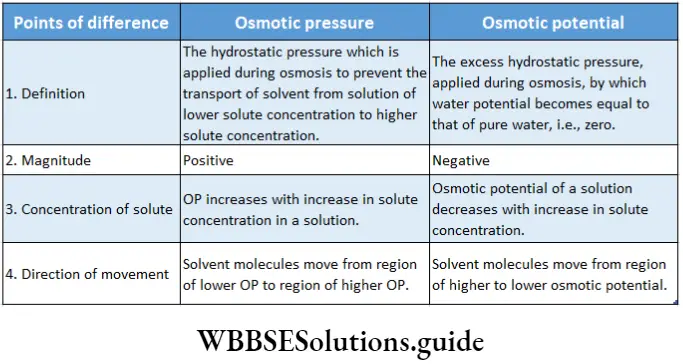
What will happen if a 10% sucrose solution and 2% sucrose solution or pure water is separated by a parchment paper?
” osmosis definition”
Parchment paper is a semipermeable membrane. Water molecules can pass through it but, sucrose molecules cannot. 10% sucrose solution is more concentrated than 2% sucrose solution. So, when the solutions are
” osmosis and osmotic pressure “
Separated by a semipermeable membrane like parchment paper, water molecules will move from 2% sucrose solution to 10% sucrose solution. This process will continue until both solutions become isotonic.

When 10% sucrose solution and pure water are separated by parchment paper, water molecules (from its pure side) will move into 10% sucrose solution. This process will continue until both solutions become isotonic. However, this process will take a longer time than the above-mentioned condition.
“osmosis definition and examples in daily life”
Type of solutions on the basis of concentration of cell sap
1. Hypertonic solution: When the concentration of the external aqueous medium is more than the concentration of cell sap, the external solution is called hypertonic solution with respect to the cell sap. Therefore, OPe > OPi. [Where OPe = osmotic pressure of the external solution, and OPi – osmotic pressure of cell sap] When a cell is placed in a hypertonic solution, water moves out of the cell and the cell shrinks (plasmolyzed).
2. Isotonic solution: When the concentration of the external aqueous medium is equal to the concentration of cell sap, the external solution is called isotonic solution with respect to the cell sap. Therefore, OPe = OPi. When a cell is placed in isotonic solution it doesn’t undergo any change.
| Class 11 Biology | Class 11 Chemistry |
| Class 11 Chemistry | Class 11 Physics |
| Class 11 Biology MCQs | Class 11 Physics MCQs |
| Class 11 Biology | Class 11 Physics Notes |
“importance of osmosis in plants and animal cells”
3. Hypotonic solution: When the concentration of the external aqueous medium is less than the concentration of cell sap, the external solution is called the hypotonic solution with respect to the cell sap. Therefore, OPe > OPi. When a cell is placed in a hypotonic solution, the cell swells and becomes turgid. If a plasmolysed cell is placed in a hypotonic solution it will regain its turgidity (deplasmolysation).
“osmosis types “

The process of osmosis occurring between a cell and its exterior is of four types.
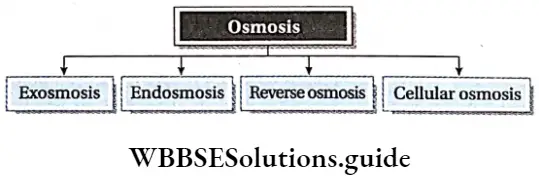
Exosmosis : When the water from cell sap or from an intact cell moves out due to hypotonic external solution, it is called exosmosis. This causes shrinkage of cytoplasm and protoplasm which results in a decrease in the volume of the cell.
Exosmosis Example: When a plant cell with a primordial utricle is placed in a hypertonic solution, water from vacuoles moves out into the extracellular fluid by exosmosis.
Endosmosis: When water moves into the cell due to lower solute concentration in an external solution (hypotonic), it is called endosmosis. This causes an increase in the volume of cells.
“types of osmosis: endosmosis and exosmosis explained”
Endosmosis Example: Terrestrial plants absorb capillary water through root hairs by the process of endosmosis.
Cell-to-cell osmosis: In multicellular organisms, metabolism in living cells requires cell-to-cell transport of substances, across the membranes. This stepwise osmosis is called cell-to-cell osmosis.
Cell-to-cell osmosis Example: Multicellular cortex region of the roots is made up of living parenchyma cells. Water absorbed by root hairs reaches the endodermis through the cortex by cell-to-cell osmosis.
Reverse osmosis: The process by which solvent particles are compelled to move by exerting pressure from a concentrated (higher solute concentration) to a dilute solution (lower solute concentration), across a semipermeable membrane, is called reverse osmosis.
Reverse osmosis Example: Processes like the thickening of fruit juices and the preparation of pure drinking water from saline water are done by the process of reverse osmosis.
Demonstration of Osmosis
The process of osmosis can be demonstrated through various experiments.
Test for Exosmosis: The process of exosmosis is explained below with the help of an experiment.
Demonstration of Exosmosis Materials required: Some fresh grapes, concentrated sugar solution, glass Petri dish.
Demonstration of Exosmosis Procedure: The fresh grapes are placed in a glass Petri dish and a concentrated sugar solution is added to it. The arrangement is kept undisturbed for some hours.
“define osmosis in biology “
Demonstration of Exosmosis Observation: After a few hours, it is observed that the grapes have shrunk.
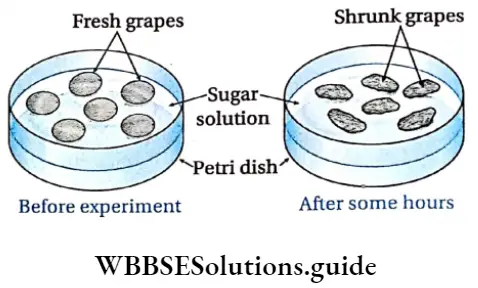
Demonstration of Exosmosis Inference: Concentrated sugar solution acts as a hypertonic solution. When fresh grapes are placed in it, the water in the grapes moves out into the sugar solution by exosmosis, causing them to shrink.
Test for Endosmosis: The process of endosmosis is explained below with the help of an experiment.
Endosmosis Materials required: A few raisins, pure water, glass Petri dish.
Endosmosis Procedure: A few raisins are placed in a glass Petri dish and pure water is added to it. The arrangement is kept undisturbed for some hours.
Endosmosis Observation: After a few hours, it is observed that the raisins in the Petri dish have become swollen.
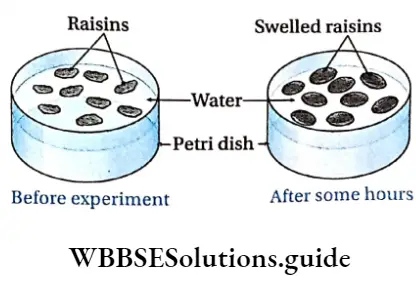
Endosmosis Inference: Pure water is hypotonic to dried raisins. So, when raisins are placed in it, water enters raisins by endosmosis which causes them to swell.
Experiment on Osmosis by Thistle Funnel:
The process of osmosis is explained below with the help of an experiment using a thistle funnel.
“reverse osmosis process and its applications in water purification”
Materials required: A large beaker, one thistle funnel, sugar, stand and clamp, cellophane membrane (semipermeable membrane), eosin dye, pure water, thread, and glass marker.
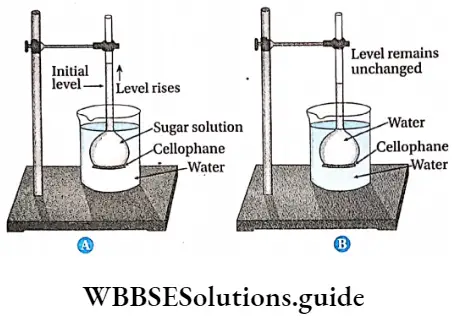
Osmosis by Thistle Funnel Procedure:
- The sugar solution is prepared using pure water and sugar.
- A thistle funnel is taken and its mouth is covered by cellophane membrane.
- The thistle funnel is fixed to a stand in an inverted position (i.e., mouth facing downward). The sugar solution is added to the thistle funnel and its level is marked by a glass marker.
- The glass beaker is filled with 2/3rd parts with water. A few drops of eosin dye is added to it. The water turns red due to this dye.
- This beaker is then kept below the inverted thistle funnel in such a way that the mouth of the thistle funnel remains immersed in the beaker.
- The arrangement is kept undisturbed for a few hours.
Osmosis by Thistle Funnel Observation: After a few hours it is observed that the level of the solution in the thistle funnel has increased and it has become red.
Osmosis by Thistle Funnel Inference with explanation: The solution in the thistle funnel is hypertonic to the water in the beaker. Since the cellophane membrane acts as a semipermeable membrane, so water from the beaker moves into the thistle funnel by endosmosis. As a result, the level of solution in the thistle funnel increases, and it gradually turns red. This proves that osmosis has taken place.
“role of osmotic pressure in maintaining cell shape”
Experiment on osmosis by Potato-Osinoscope: The process of osmosis is explained below with the help of a potato-osmoscope.
osmosis by Potato-Osinoscope Materials required: A large fresh potato, concentrated sugar solution, pure water, a large glass beaker, knife, a few pins, and eosin dye.
osmosis by Potato-Osinoscope Procedure:
- The large potato is cut with a knife in a cube shape so that it can be well placed in the beaker. Some portion of the potato is scooped out from the middle, such that a cavity is formed within the potato. This is used as a potato osmoscope.
- This cavity in the osmoscope is filled with concentrated sugar solution, The level of the solution is marked by pricking a pin at that level.
- This arrangement is carefully placed in the beaker. The beaker is filled with pure water in such a way that part of the potato cube should remain immersed in water.
- A few drops of eosin dye is added to the water in the beaker.
- The total arrangement is kept undisturbed for a few hours.
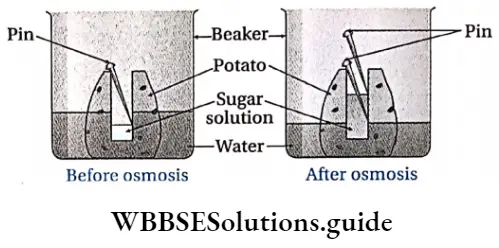
osmosis by Potato-Osinoscope Observation: After a few hours, it is observed that the level of the solution in the cavity of the potato cube has increased and turned red. This level is marked again with a pin.
Inference with explanation: The sugar solution in the potato is hypertonic to the water in the beaker. So, pure water from the beaker enters the cavity of the potato osmoscope by endosmosis. As a result, the level of solution within the cavity of the potato cube increases and it turns red.
Experiment on Osmosis by Egg-Osmoscope: The process of osmosis is explained below with the help of a egg-osmoscope.
Osmosis by Egg-Osmoscope Materials required: An egg, dilute HCI, thin glass tube, glass marker, cone, sugar solution, stand and clamp, sealing wax, one beaker, pure water, and eosin solution.
Osmosis by Egg-Osmoscope Procedure:
- A small pore is made in the shell of an egg gently and its content is removed. The opposite end of the egg is immersed in dilute HCI. As a result, the hard outer shell of the egg gets dissolved in HCI, but the thin inner membrane remains intact. Now, the eggshell is fixed to a thin glass tube at the site of the pore and sealed with sealing wax. This arrangement is called an egg osmoscope.
- The egg osmoscope is fixed to a stand with the help of a clamp. The concentrated sugar solution is poured into the glass tube. The level of sugar solution is marked with a glass marker.
- A beaker is taken and filled with pure water. A few drops of eosin dye is added to it. The beaker is kept below the egg osmoscope in such a way that half of the egg remains immersed in pure water. This arrangement is kept undisturbed to stand for a few hours.
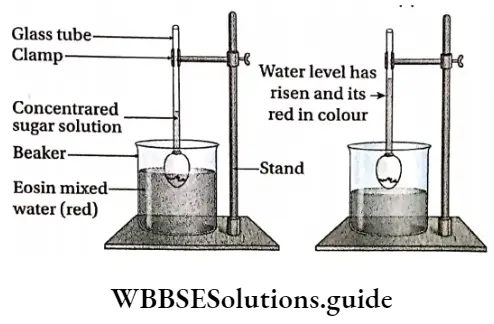
Osmosis by Egg-Osmoscope Observation: After a few hours, it is observed that the level of sugar solution in the egg osmoscope has increased and it has turned red.
Inference with explanation: The inner layer of the egg is semipermeable. The solution in the egg osmoscope is more concentrated than the pure water outside. So, by endosmosis, water from the beaker enters the egg through the semipermeable membrane and turns the solution red.
Osmotic Pressure or OP
Osmotic Pressure Definition: The opposite hydrostatic. pressure which is applied to a solution to prevent the entry of the solvent molecules from a solution of lower solute concentration to a solution of higher solute concentration, when the two are separated by a semipermeable membrane, is called osmotic pressure.
The osmotic pressure of a solution is denoted by the Greek letter pi (π).
The osmotic pressure is directly proportional to the solute concentration of the solution.
The term ‘osmotic pressure’ is ambiguous. An isolated solution cannot possess osmotic pressure since the phenomenon involves a system, containing both pure solvent and solution separated by a semipermeable membrane. Therefore, it is confusing to refer to this pressure in an isolated solution, although the solution possesses some osmotic pressure.
“MCQs on osmosis and osmotic pressure with answers”
Due to this reason, the term osmotic potential or solute potential (ψs) is used instead of osmotic pressure. It is equal in magnitude but opposite in sign to n. Thus, π = – ψs The osmotic pressure of pure water is zero. This is due to the absence of solute in pure water.
- The lowest osmotic pressure is found in cells of aquatic plants or hydrophytes.
- The highest osmotic pressure is found in cells of a halophytic plant named Atriplex confertifolia which is p ft* C T approximately 202.5 atmospheres.
- Generally, osmotic pressure is less during the night and higher at noon.
- Osmotic pressure is expressed in terms of atmosphere or bar.
- Osmotic pressure is calculated by the following formula, given by Vant Hoff.
- At normal or standard temperature and pressure (STP), the osmotic pressure of 1 molar solution is 22.4 bar and the osmotic potential is -22.4 bar.
(ψs) = n/V RT
n = number of solute particles in solution of volume V
V = volume of solution
R = gas constant
T = specific temperature [273 +………..OC]
Factors influencing Osmotic Pressure: Several factors regulate osmotic pressure, such as
Osmotic Pressure Concentration of the solute: The osmotic pressure (OP) of a solution is directly proportional to the molar concentration of solutes.
The molecular weight of solute: OP of a solution is inversely proportional to the molecular weight of its constituent solute. It means, the higher the molecular weight of the solute, the lower will be the OP.
Osmotic Pressure Temperature: The osmotic pressure of a solution is directly proportional to the temperature of the medium. It means that the osmotic pressure of the solution increases an increase in temperature.
Osmotic Pressure Ionization of solute: Dissociation of solutes into ions (ionization) also increases the OP of a solution.
Significance of osmosis
- Root hairs of terrestrial plants absorb water from the soil by the process of endosmosis. The cells present in root hairs of plants have a single large, central vacuole. The cell sap within that vacuole is more concentrated than the water in the soil. This causes the water to enter the cells by endosmosis.
- The movement of water in plants through the cortex to the endodermis is performed by cell-to-cell osmosis. Due to endosmosis, the turgor pressure of cells increases which creates root pressure in the endodermis. Root pressure helps in the ascent of sap through xylem vessels. The distribution of water in plants also takes place by osmosis.
- Turgidity develops due to endosmosis. It helps to maintain a definite shape of tender parts like leaves, stems, and flowers. Turgidity also provides mechanical strength to the plants.
- The opening and closing of stomata also depend upon the process of osmosis.
- Movement in plants is dependent on osmosis. example
The leaves of Mimosa pudica droop down due to a sudden change of turgor pressure in the cell sap of cells in the petiole of the leaf. This is related to osmosis.
Dehiscence of fruits and sporangium dependent on the process of osmosis,
Rapid movement of the leaves of Desmodium (telegraph plant) is also due to osmosis. - The resistance against dryness is increased due to high osmotic concentration.
- Turgor pressure, especially in meristematic tissue helps in cell elongation.
Different Pressures Related to Osmosis
Different osmosis-related pressures are described below.
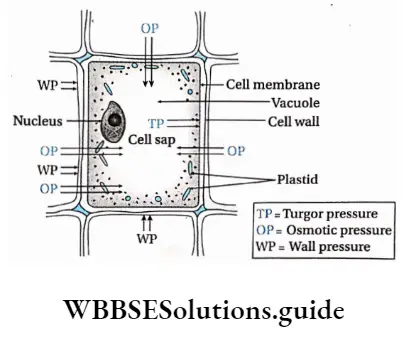
Hydrostatic Pressure: The pressure applied by absorbed water on the cell membrane of a turgid plant cell, when kept in a hypotonic solution, is called hydrostatic pressure or HP.
Suction Pressure: The difference of diffusion pressure between molecules of solute present outside and inside of a cell separated by semipermeable membrane is called suction pressure or SP. It can be calculated as, SP = OP-TP.
Wall Pressure: The equal and opposite pressure exerted by the cell wall against the turgor pressure in a fully turgid cell is called wall pressure or WP. Hence, TP = -WP, in a fully turgid cell.
Osmotic Pressure: The minimum, external pressure which is required to stop the net movement of water across the semipermeable membrane in a system, is called osmotic pressure or OP. Its value is positive. OP is zero in pure water. It increases with an increase in solute concentration in a solution. It can be calculated as, OP = TP + SP. Water molecules move towards the solution with higher osmotic pressure from the lower osmotic pressure across the semipermeable membrane.
“diagram-based explanation of osmosis in cells”
Turgor Pressure: The pressure exerted on the cell wall due to endosmosis of water is called turgor pressure or TP. A flaccid cell has zero turgor pressure. The highest value of turgor pressure is found in turgid cells and it is equal to the osmotic pressure. In a fully turgid cell OP = TP.
Nowadays, turgor pressure is known as pressure potential and it is represented as Ψp.
Diffusion Pressure Deficit: The difference between the diffusion pressure of pure solvent and its solution is called its diffusion pressure deficit or DPD. It can be calculated by the formula—
DPD = OP- WP (or TP)
Its value is positive.
Interrelationship among osmotic pressure, turgor pressure, wall pressure, and diffusion pressure deficit
1. When a mature plant cell is kept submerged in pure water, water enters the cell vacuole due to endosmosis. This happens because OPe = OPi (here, the letter ‘e’ denotes the exterior of the cell and T denotes the interior of the cell). This in turn causes the cell to swell up. When a cell is fully turgid, then OPe = OPi.
2. When a cell is immersed in water, molecules of water enter the cell due to the high osmotic pressure of the cell sap. This produces turgor pressure in the cell. The turgor pressure is counterbalanced by an equal and opposite pressure exerted by the cell wall, known as wall pressure. Therefore, wall pressure and turgor pressure become equal but opposite in magnitude to each other, TP = -WP.
If immersed in a hypotonic solution, a plant cell remains intact. However, an animal cell can burst under such conditions. Due to the lack of cell walls, there is no wall pressure to counter the turgor pressure as endosmosis occurs.
3. Plants intake water from the soil by endosmosis through root hairs. In a turgid cell,’ the hydrostatic pressure acting per unit volume is called suction pressure. Water enters root hairs due to diffusion pressure deficit within root hairs. The more the DPD in root hairs, the more the suction pressure. This, in turn, will increase the rate of absorption of water. Thus, SP is dependent on DPD.
4. Again, DPD is equal to the difference between osmotic potential and turgor pressure.
DPD = ψs -TP
A plant cell as an osmotic system—relation between water potential, osmotic potential, and pressure potential
When a typical plant cell is kept immersed in pure water or solution, it acts as an osmometer (device for demonstrating osmosis). The plant cell wall is made up of cellulose. It is permeable in nature and has tensile strength (resist breaking under tension).
A mature plant cell contains a centrally located large vacuole in the cytoplasm. It is surrounded by a thin layer of cytoplasm, called tonoplast. Cell sap is present within the vacuole. It keeps the cell in proper shape. When endosmosis occurs, the cell becomes turgid. But, when exosmosis occurs, the cell becomes flaccid.
In the case of a turgid cell: The transport of water stops in a fully turgid plant cell. In that case, osmotic potential and pressure potential are equal in magnitude but opposite in direction.
Suppose, in a fully turgid cell, the osmotic potential is = ψs = -10 bar
Therefore, Ψw=ψs+Ψp = -10 bar + 10 bar = 0
Thus, Ψw = 0
“difference between osmosis and diffusion with examples”
In case of a flaccid cell: In a flaccid cell, TP = 0. In such a cell, the magnitude of ψs and Ψw is the same. Suppose, in the same plant cell,ψs = -10 bar and Ψp = 0 bar
Therefore, Ψw=ψs+Ψp
= -10 bar + 0 bar = -10 bar
Thus, water potential is equal to osmotic potential. Water potential in such a cell is less than pure water (Ψw= 0).
Therefore, water will always move from a region of higher water potential to a region of lower water potential