Periodic Properties
Most of the properties of atoms (elements), such as valency, ionisation enthalpy and electron affinity depend upon their electronic configuration. Elements of the same group have the same valence shell configuration, so they exhibit similar chemical properties. However, atomic size increases down a group, so the reactivity of elements belonging to the same group varies down the group.
Valency: The chemical properties of an element depend upon the number of electrons present in the outermost shell (valence shell) of its atoms. These electrons, called valence electrons, determine the valency of the atom.
The valency of an element is defined as its combining capacity. It is often expressed in terms of the number of hydrogen atoms, chlorine atoms, or double the number of oxygen atoms that one atom of the element combines with. For the representative elements, valency is either equal to the number of valence electrons or eight minus the number of valence electrons (if the number of valence electrons is five or more). The transition elements show variable valency.
Read and Learn More WBCHSE For Class11 Basic Chemistry Notes
Variation of valency in a period The number of valence electrons in representative elements increases from 1 to 8 while moving across a period. For s-block elements, the group number is the same as the number of valence electron (1 or 2), so valency is the same as the group number. For p-block elements, the number of valence electrons is the group number minus ten.
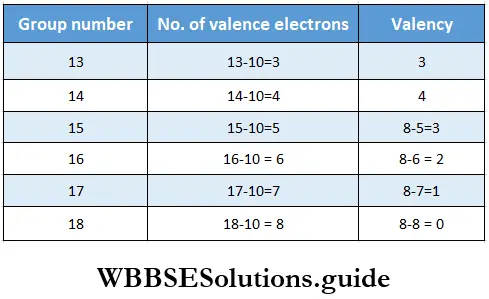
Variation of valency in a group The number of valence electrons is the same for all the elements of a group, so members of the same group exhibit the same valency.
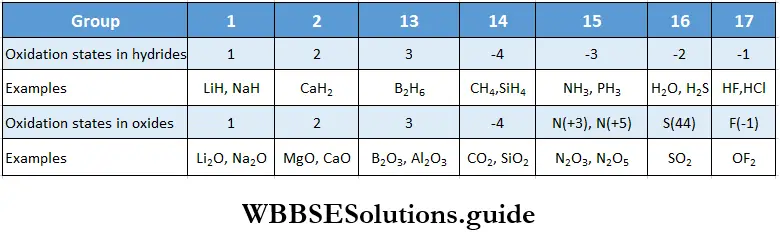
Valencies of the elements show some periodic trends. Transition elements (Groups 3 to 12) exhibit variable valencies and oxidation states. The oxidation state is a measure of the electron control that an atom has in a compound compared to the atom in a pure element. Periodic trends in the oxidation states of the elements of Groups 1 and 2 (s block) and Groups 13 to 17 (p block) are shown in Table with the help of the formulae of their hydrides and oxides.
Atomic size: For the purpose of determining its size an atom is considered to be a sphere, and the atomic radius is the radius of this sphere. The atomic radius is defined as the distance from the centre of the nucleus of the atom to the outermost shell of electrons. It can also be defined as the distance from the centre of the nucleus to the point up to which the density of the electron cloud (or the probability of finding electrons) is the maximum.
However, it is not possible to determine the atomic radius precisely because of the following reasons.
- It is not possible to isolate an atom.
- According to the wave mechanical model of the atom, the probability of finding an electron is never zero, even at a very large distance from the nucleus. In other words, it is not possible to define the boundary of an atom.
- The probability distribution of the electrons, of an atom is affected by the presence of other atoms in its neighbourhood.
- The atomic radius changes from one bonding state to another. This is the reason why atomic radii may be given different operational names, like covalent radius, van der Waals radius and metallic radius.
Covalent radius The approximate radius of an atom can be determined by measuring the distance between two atoms in a covalent molecule. This is done by X-ray diffraction and other spectroscopic techniques. The covalent radius of an atom may be defined as one-half the distance between the centres of the nuclei of two similar atoms bonded by a single covalent bond.
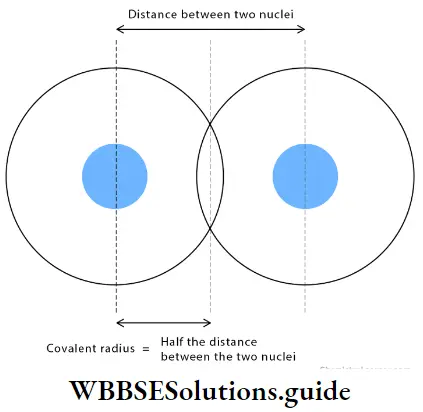
For a homonuclear diatomic molecule, for example, chlorine (Cl2), half the distance between the centres of the two chlorine atoms is the covalent or atomic radius of the chlorine atom, which is equal to 99 pm.
rcovalent = 1/2[intemuclear distance between two bonded atoms]
Vander Waals radius This is defined as half the distance between the centres of the nuclei of the two closest nonbonded atoms of adjacent molecules of an element in the solid state.
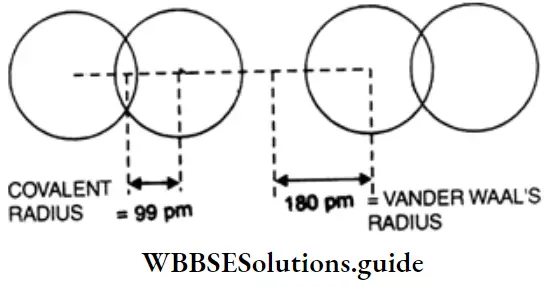
Obviously, the van der Waals radius of an atom depends upon the packing of the atoms when the element is in the solid state. The van der Waals radius of an atom is greater than its covalent radius because the formation of a covalent bond involves the overlapping of the orbitals of two atoms, which decreases the internuclear distance.
Metallic radius The metallic radius of an atom in a metallic crystal is half the distance between the centres of the nuclei of two adjacent atoms in the metallic crystal. The metallic radius of an atom is greater than its covalent radius because the metallic bond is weaker than the covalent bond, so the internuclear distance between adjacent atoms in a metallic crystal is greater than the internuclear distance between two atoms held by a covalent bond.
Variation of atomic radius in a period The atomic radius decreases with increase in atomic number across a period. This is because the nuclear charge increases as the atomic number increases, say by one unit, but the extra electron is added to the same principal shell. The electron cloud is pulled closer to the nucleus by the increased nuclear charge so the atomic radius becomes smaller. Table shows the variation in atomic radius across the second period.
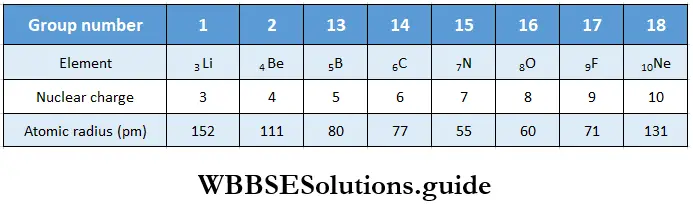
As you can see from the table, the atomic radius decreases across the period and then there is an abrupt increase in the atomic radius of neon. This is because while for the other elements the values represent the covalent radii, the value for Ne represents its van der Waals radius (Ne is an inert gas).
Variation of atomic radius in a group In general, the atomic radius increases as one moves down a group. This is because though the nuclear charge increases, the effect of the extra charge is not as pronounced as the effect of the additional shell of electrons.
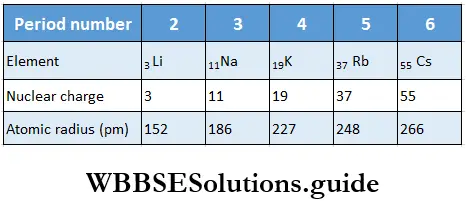
With the addition of each electron shell (increase in principal quantum number), the distance of the outermost electrons from the nucleus increases, i.e., the atomic radius increases. This is observed in Table.
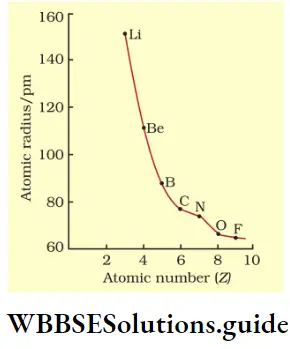
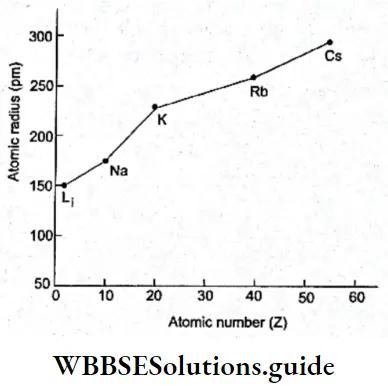
Ionic radius: A neutral atom becomes a cation when it loses one or more electrons and an anion when it gains one or more electrons. The ionic radius of an atom is the radius it exhibits in an ionic crystal like KCl in which the K+ and Cl– ions are packed together with their outermost shells of electrons in contact with each other.
- A cation has the same number of protons as the neutral atom, but fewer electrons than it. So, the nuclear influence over the electrons is greater in the cation than in the neutral atom. Consequently, the size of the cation is smaller than that of the parent atom.
- Quite often the formation of a cation involves the removal of the valence shell altogether. Obviously this means a reduction in size, as in the formation of Na+ from Na. The metallic radius of Na is 186 pm, while the ionic radius of Na+ is 102 pm.
- An anion, on the other hand, has the same number of protons as the parent atom, but a greater number of electrons than it. Since the same nuclear charge acts on a greater number of electrons, the effective nuclear charge per electron decreases, and the electron cloud is held less tightly by the nucleus. Consequently, the anion is bigger than the parent atom.
- Take, for example, the case of the chloride ion which has 18 electrons, one electron more than the chlorine atom. Its size is 184 pm while the size of the parent atom is 99 pm.
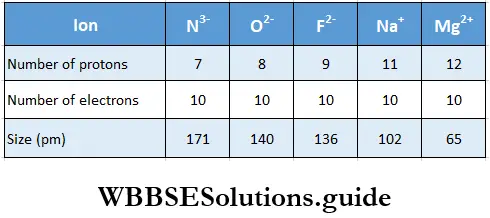
- Isoelectronic ions Ions with the same number of electrons but different magnitudes of nuclear charge are called isoclectronic ions. These are really ions of different elements with the same electronic configuration, example, Cl– and K+ both have 18 electrons but the former has 17 protons, while the latter has 19 protons.
- Within a series of isoelectronic ions, as the nuclear charge increases, the influence of the nucleus over the electrons increases, so the ionic radius decreases. That is to say, the size of isoelectronic ions decreases with increase in effective nuclear charge.
- We may also conclude that the cation with greater positive charge will have smaller radius. Anions with a greater negative charge will be larger in size as tire net repulsion of electrons will counteract the nuclear charge.
Trends in noble gas radii: Noble gases ordinarily do not form covalent bonds. In crystals of noble gases, therefore, no chemical forces operate between the atoms. The van der Waals forces are the only attractive forces between the atoms of these elements. Hence the atomic radii of noble gases are van der Waals radii, which represent the distance of closest approach of the two adjacent atoms in the solid state.
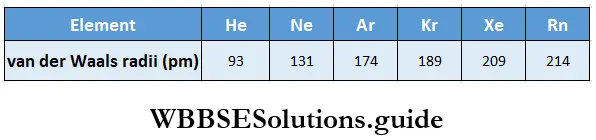
The van der Waals radii of noble gases are, therefore, much larger than the covalent radii of the corresponding adjacent elements.
Ionisation enthalpy: Energy is required to promote an electron from lower energy level to higher energy level, but if the amount of energy supplied is sufficiently large the electron may be completely removed. This energy is referred to as ionisation enthalpy. Defined in a more formal way, ionisation enthalpy is the enthalpy change accompanying the removal of an electron from a gas-phase atom.
⇒ \(A(g)+\text { Energy }(\text { IE }) \longrightarrow A^{+}(\mathrm{g})+\mathrm{e}^{-}\)
or, \(\mathrm{A}(\mathrm{g})\longrightarrow \mathrm{A}^{+}(\mathrm{g})+\mathrm{e}^{-} ; \Delta H\)
Ionisation enthalpy is also called ionisation potential because it is the potential required to remove the electron from the gaseous atom. It is expressed either in electronvolts per atom or kilojoules per mole of atoms.
∴ 1 eV = 96.3 kJ mol-1
Energy is always required to remove electrons from an atom and hence ionisation enthalpies are always positive.
Successive ionisation enthalpies The amounts of energy required to remove the second, third and fourth electrons from the unipositive, dipositive and tripositive ions are called the second, third and fourth Ionisation enthalpy respectively. These are designated IE2, IE3 and IE4, and are collectively known as successive ionisation enthalpies.
⇒ \(\mathrm{A}(\mathrm{g})+\mathrm{IE}_1 \longrightarrow \mathrm{A}(\mathrm{g})^{+}+\mathrm{e}^{-}\)
⇒ \(\mathrm{A}^{+}(\mathrm{g})+\mathrm{IE}_2 \longrightarrow \mathrm{A}^{2+}(\mathrm{g})+\mathrm{e}^{-}\)
⇒ \(\mathrm{A}^{2+}(\mathrm{g})+\mathrm{IE}_3 \longrightarrow \mathrm{A}^{3+}(\mathrm{g})+\mathrm{e}^{-}\)
After the removal of the first electron, the atom changes into a monopositive ion which has the same number of protons as the parent atom but fewer electrons than it. Consequently, the effective nuclear charge per electron increases and more energy is required to remove the second electron. Thus, the value of the second ionisation enthalpy (IE2) is greater than that of the first (IE1). Similarly, the value of IE3 is greater than that of IE2.
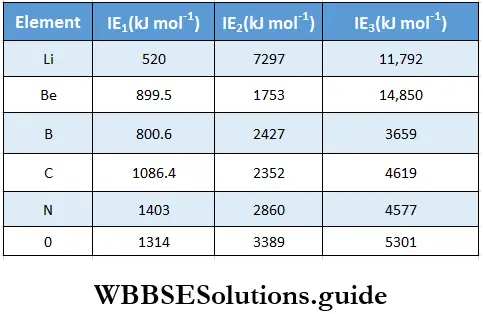
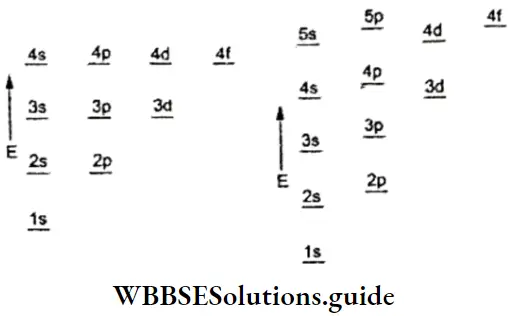
Like valency and atomic size, the ionisation enthalpy of elements exhibits certain periodicity, as is evident from figure.
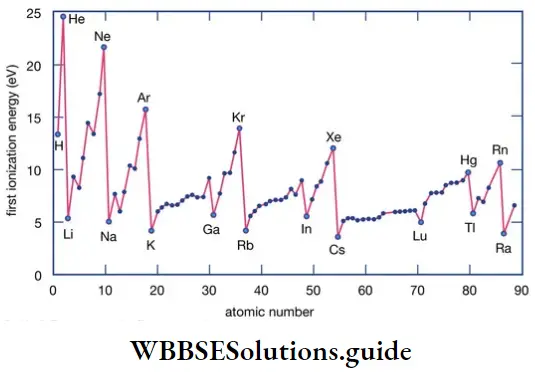
The graph in the figure shows two striking features.
- The noble gases have the highest ionisation enthalpies in their respective periods.
- The alkali metals Li, Na, K and Rb have the lowest ionisation enthalpies in their respective periods.
To understand this periodicity, let us discuss the factors upon which the ionisation enthalpy of an element depends. The ionisation enthalpy depends upon the following factors.
- Atomic size
- Magnitude of nuclear charge
- Screening effect of inner electrons
- Penetration effect of electrons
- Electronic configuration
Atomic size The ionisation enthalpy depends upon the attractive force between the electron (to be removed) and the nucleus. And this force is inversely proportional to square of the distance between them \(F=q_1 q_2 / d^2\)
Consequently, as the size of the atom increases, and the hold of the nucleus over valence electrons decreases, the ionisation enthalpy decreases. In general, ionisation enthalpy decreases down the group.
Magnitude of nuclear charge The force of attraction between the valence electrons and the nucleus increases with , the increase in the nuclear charge if the principal energy shell remains the same because the attractive force is directly proportional to the product of the charge on the nucleus and that on the electrons (F = q). In other words, ionisation enthalpy increases with increase in nuclear charge. In general, the first ionisation enthalpy increases as we go across a period.
Screening effect of inner electrons The electrons of the valence shell of an atom are attracted to the nucleus because of the nuclear charge. How much of an impact the nuclear charge has on the outermost shell of electrons depends not only on the magnitude of the nuclear charge and the distance between the nucleus and the outermost shell but also on the inner shells of electrons.
- These inner shells exert a force of repulsion on the outer shell electrons, thus acting as a shield or screen which does not let the outer electrons experience the full impact of the nuclear charge. This is called the screening effect of the inner electrons. Naturally, the screening effect depends on the number of electrons present in the inner shells and as the screening effect increases, the ionisation enthalpy decreases. This is why ionisation enthalpy decreases down a group, with the successive addition of electron shells.
- Ionisation enthalpy increases across a period because though nuclear charge increases, electrons are added to the same shell and the screening effect of the inner electrons does not increase.
Penetration effect of electrons Among all the electrons of an atom, those of the s orbital have the maximum probability of being found near the nucleus.
The probability of finding p-orbital electrons near the nucleus is much less. Similar is the case with the d- and f-orbital electrons. In other words, electrons of the s orbital are more penetrating than electrons of the p orbital. In a given shell, the penetrating power decreases in the following order.
∴ s > p > d > f
If the penetration of an electron is greater, it is closer to the nucleus and held more firmly by it. Consequently, it is more difficult to remove such an electron from the atom than a more loosely held electron. Thus, for the same shell, it is easier to remove the p electrons than the s electrons.
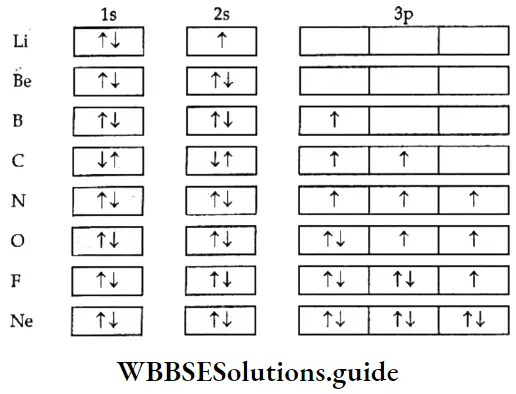
Electronic configuration Certain electronic configurations are more stable than others. Obviously, atoms which have stable configurations do not tend to lose electrons easily, so they have higher ionisation enthalpies. What makes for a stable configuration? Either completely filled orbitals or exactly half-filled orbitals of the same subshell. An atom with three half-filled p orbitals in the outermost shell is more stable than an atom with one completely filled p orbital and two half-filled p orbitals.
- The noble gases have the highest ionisation enthalpies in their respective periods because they have completely filled orbitals. The np2 ns6 configuration of noble gases is very stable.
- Take Be(1s2 2s2) and B(1s2 2s2 2p1), for example. Be with a completely filled s orbital in the outermost shell is more stable than B, which has one half-filled and two vacant p orbitals in the outermost shell. Had all three p orbitals of B been half-filled, it would have been more stable. Similarly, \(M g\left(1 s^2 2 s^2 2 p^6 3 s^2\right)\) is more stable (higher ionisation enthalpy) than \(\mathrm{Al}\left(1 \mathrm{~s}^2 2 \mathrm{~s}^2 2 \mathrm{p}^6 3 \mathrm{~s}^2 3 \mathrm{p}_x^1 3 \mathrm{p}_y 3 \mathrm{p}_z\right)\). However, if A1 had two half- filled p orbitals and one vacant p orbital, it would have been less stable than it is.
- The fact that an atom in which all the orbitals of a particular subshell are half-filled is more stable than one in which some are half-filled, while others are vacant or completely filled, becomes clear if one considers \(\mathrm{N}\left(1 \mathrm{~s}^2 2 \mathrm{~s}^2 2 \mathrm{p}_x^1 2 \mathrm{p}_y^1 2 \mathrm{p}_z^1\right) \text { and } \mathrm{O}\left(1 \mathrm{~s}^2 2 \mathrm{~s}^2 2 \mathrm{p}_x^2 2 \mathrm{p}_y^1 2 \mathrm{p}_z^1\right)\). All the p orbitals in N are half-filled, so it is more stable (higher ionisation enthalpy) than O. Similarly, the ionisation enthalpy of \(P\left(1 s^2 2 s^2 2 p^6 3 s^2 3 p_x^1 3 p_y^1 3 p_z^1\right)\) is higher than that of \(\mathrm{S}\left(1 \mathrm{~s}^2 2 \mathrm{~s}^2 2 \mathrm{p}^6 3 \mathrm{~s}^2 3 \mathrm{p}_x^2 3 \mathrm{p}_y^1 3 \mathrm{p}_z^1\right)\).
Variation in IE across a period In general, ionisation enthalpy increases across a period. This is because the nuclear charge increases but the electrons are added to the same principal shell, or the principal quantum number. remains the same. Consequently, the atomic size decreases and the valence electrons are held more tightly.
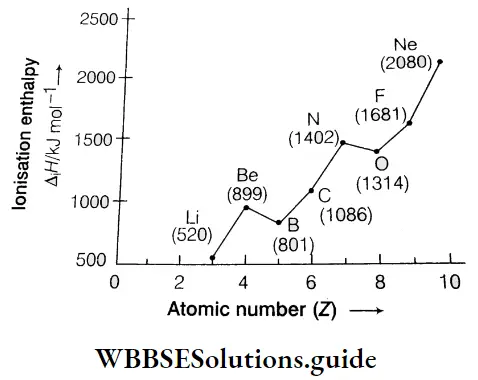
However, there are some exceptions to this rule. Let us consider the first ionisation enthalpies of the elements of the second period. They are in the following order.
3Li < 4Be > 5B< 6C< 7N> 8O < 9F < 10Ne
Irregularities in the curve occur at two points. The ionisation enthalpy of B is lower than that of Be and the ionisation enthalpy of O is lower than that of N. The ionisation enthalpy of B is lower than that of Be though B has a higher nuclear charge than Be. This is due to the following reasons.
- The electronic configuration of \({ }_5 \mathrm{~B}\left(1 \mathrm{~s}^2 2 \mathrm{~s}^2 2 \mathrm{p}^1\right)[latex] is less stable than that of [latex]4 { }_4 \mathrm{Be}\left(1 \mathrm{~s}^2 2 \mathrm{~s}^2\right)\), which has a completely filled s orbital in its outermost shell.
- The outermost electron of B belongs to the 2p orbital while the outermost electrons of Be belong to the 2s orbital. The penetrating power of s electrons is more than that of p electrons, so it takes less energy to remove an electron from the p subshell than from the s subshell. Hence, the first ionisation enthalpy of Be is more than that of B.
N(1s2 s2 2p3) has a more stable configuration than O(1s2 2s2 2p4), so there is a slight decrease in ionisation enthalpy from N to O.
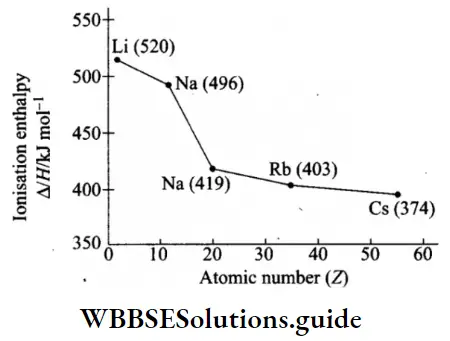
Variation in IE down a group Ionisation enthalpy decreases regularly down a group. This can be explained in terms of increasing atomic size and the screening effect of inner shells.
- The atomic size increases down a group because of the addition of new principal shells, which means that the distance between the nucleus and the valence electrons increases, hence the attractive force exerted by the nucleus on the outermost electrons decreases. This leads to the lowering of ionisation enthalpy.
- The addition of new shells also means that the screening effect increases, or the force of attraction felt by the valence shell electrons decreases. This is the second factor which lowers the value of ionisation enthalpy.
- The nuclear charge increases as the atomic number increases. This should lead to an increase in ionisation enthalpy down a group.
- However, the screening effect more than compensates for the impact of the increased nuclear charge. In case of alkali metals, shielding is the most effective as the orbitals in the inner shells are completely filled.
Electron gain enthalpy (Δeg H): The electron gain enthalpy of an atom is a measure of its tendency to form an anion or a measure of the firmness with which an extra electron is bound to it. It is defined as the enthalpy change accompanying the process when an electron is added to a neutral gaseous atom to form a monovalent anion.
⇒ \(\mathrm{X}(\mathrm{g})+\mathrm{e}^{-} \longrightarrow \mathrm{X}^{-}(\mathrm{g}) ; \Delta H=\Delta_{\mathrm{eg}} H\)
- The more negative the electron gain enthalpy of an atom, the its greater is tendency to accept to an electron gain enthalpy is expressed in eV per atom or kJ mol-1.
- The process of electron gain can be endothermic or exothermic, depending on the nature of the element, and so the values of electron gain enthalpy may be positive or negative respectively. In case of electronegative elements, energy is released when one electron is added to a neutral gaseous atom.
After the addition of one electron, the atomf becomes negatively charged and the addition of a second electron is opposed by the electrostatic force of repulsion between the uninegative anion and the electron which is to be added. So, energy has to be supplied for the addition of the second electron. Thus, the second electron gain enthalpy is positive, and so is the third, and so on.
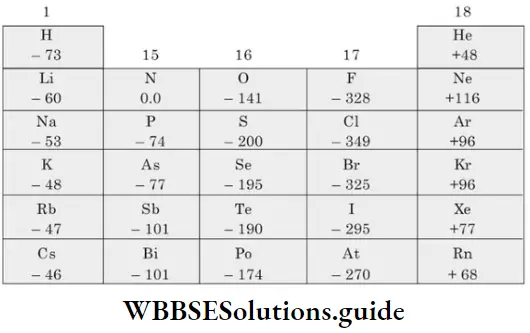
⇒ \(\mathrm{O}(\mathrm{g})+\mathrm{e}^{-} \longrightarrow \mathrm{O}^{-}(\mathrm{g}) ; \Delta_{\mathrm{eg}} H_1=-141 \mathrm{~kJ} \mathrm{~mol}^{-1}\)
⇒ \(\mathrm{O}^{-}(\mathrm{g})+\mathrm{e}^{-} \longrightarrow \mathrm{O}^{2-}(\mathrm{g}) ; \Delta_{\mathrm{eg}} H_2=+844 \mathrm{~kJ} \mathrm{~mol}^{-1}\)
The electron gain enthalpy of an atom depends on the following factors.
Atomic size The distance between the nucleus and the outermost shell (which receives additional electrons) increases as atomic size increases. Consequently, the force of attraction between the nucleus and the additional electron decreases and the electron gain enthalpy becomes less negative.
Nuclear charge The force of attraction between the nucleus and the additional electron increases with increase in nuclear charge. With the increase in nuclear charge, electron gain enthalpy becomes more negative.
Electronic configuration Elements which have completely filled orbitals or exactly half-filled orbitals (of the same subshell) are more stable than other elements. Such elements do not accept additional electrons readily, so their electron gain enthalpies have large positive values.
The variation of electron gain enthalpy across a period or down a group is not absolutely regular. However, there is a general trend, despite some exceptions.
Variation of Δeg H in a group Both the atomic size and the nuclear charge increase down a group. But the effect of the increase in atomic size is more pronounced. Thus, the attractive force experienced by an additional electron decreases as atomic number increases and electron gain enthalpy is less negative down a group. For example, the Δeg H of bromine is greater than that of chlorine.
- However, the electron gain enthalpies of some elements of the second period, like O and F, are less negative than the electron gain enthalpies of the corresponding elements S and Cl of the third period. This is because the extremely small size of these atoms leads to the presence of considerable electron-electron repulsions within these atoms (of the elements of the second period).
- This electronic repulsion more than offsets the impact of the smaller atomic size and opposes the addition of an electron. In other words, though the smaller size should have led to greater nuclear attraction for an additional electron, the interelectronic repulsion actually makes the atom less ready to accept an electron. The AegH of oxygen (-141 kJ mol-1) is less negative than that of sulphur (-200 kJ mol-1).
Variation of Δeg H in a period In general, electron gain enthalpy becomes more negative across a period. This is because the atomic size decreases, while the magnitude of the nuclear charge increases. This increases the nuclear attraction experienced by an additional electron or makes the atom more ready to accept an electron.
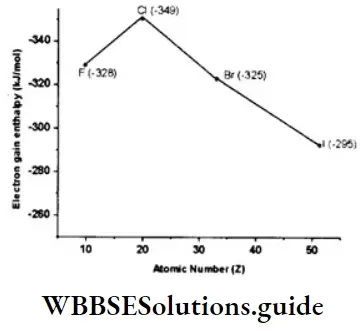
- Halogens have the most negative electron gain enthalpies in their respective periods. This is because halogens (ns2np5) have seven electrons in the valence shell.
- They require only one electron to acquire the stable configuration of eight electrons in the outermost shell, so they have a strong tendency to accept an electron. They also have the smallest size in their respective periods. This, too, makes them more ready to accept an electron than other members of their period.
- The value of electron gain enthalpy becomes less negative from chlorine to iodine because of increasing atomic radius. However, as already explained, the electron gain enthalpy of F is unexpectedly less negative than that of Cl.
Δeg H of elements with completely filled or exactly half-filled orbitals
- The alkaline earth metals, or elements of Group 2, have positive electron gain enthalpies. This is due to the fact that the s subshell of these elements is completely filled. This makes them very stable and they do not have a tendency to accept electrons.
- Nitrogen and phosphorus have very low electron gain enthalpies because they have stable valence shell configurations with exactly half-filled p orbitals.
- The electron gain enthalpy of noble gases is high and positive. Their valence-shell configuration is extremely stable with completely filled subshells (ns2 np6). Any additional electron would have to be placed in an orbital of the next higher energy shell. The shielding effect of the inner electrons and the large distance from the nucleus makes the addition of an electron impossible.
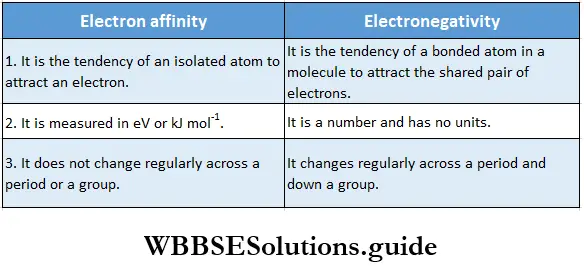
The term electron gain enthalpy is closely related to electron affinity. Electron affinity, Eea, is the energy released when an electron is added to a neutral gaseous atom. It is taken as positive when energy is released and negative when energy is absorbed, in contrast to the thermodynamic convention. Thus, electron affinity and electron gain enthalpy have very similar numerical values but differ in sign.
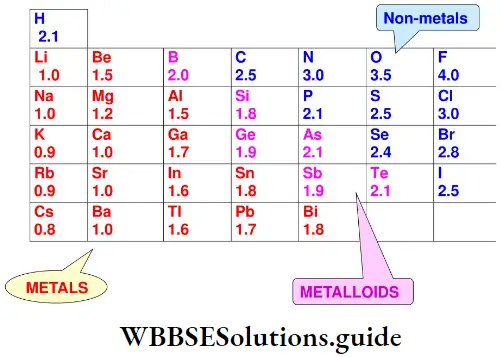
Electronegativity: The electronegativity of an element is a qualitative measure of the ability of its atom to attract the pair of electrons shared by bonded atoms in a molecule. Linus Pauling formulated a numerical scale of electronegativity based on considerations of bond dissociation energies. Table lists values for the main-group elements.
An atom that has a high electronegativity is likely to be one having a high ionisation enthalpy (so that it is unlikely to lose electrons to another atom in the molecule). Also, such an atom will have a high negative electron gain enthalpy so that it is energetically favourable for an electron to move towards it.
Electronegativities show a periodicity and the elements with the highest electronegativities are those close to fluorine in the periodic table. Fluorine is the most electronegative element while caesium is the least electronegative element.
Variation of electronegativity in a period In a period, in general, electronegativity increases from left to right. This is because the attraction between the valence electrons and the nucleus increases as the atomic radius decreases across a period.
The increase in electronegativity across a period is directly related to the increase in nonmetallic s’- properties of elements when we move from left to right across a period. You already know that nonmetals have the tendency to accept electrons.
Variation of electronegativity in a group The electronegativity values decrease with the increase in atomic size down a group. For example, the electronegativity of fluorine is the most and that of the iodine is the least in Group 17.

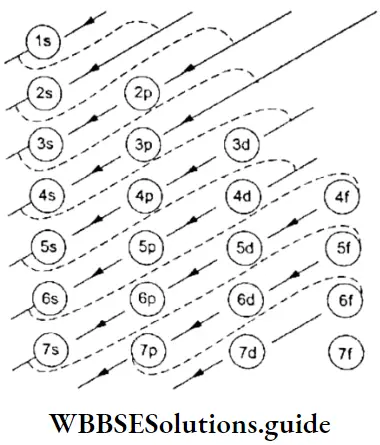
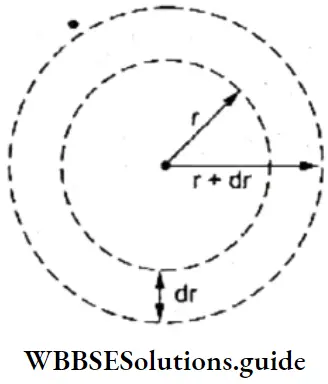
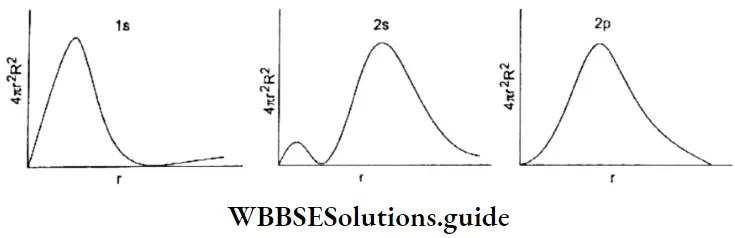
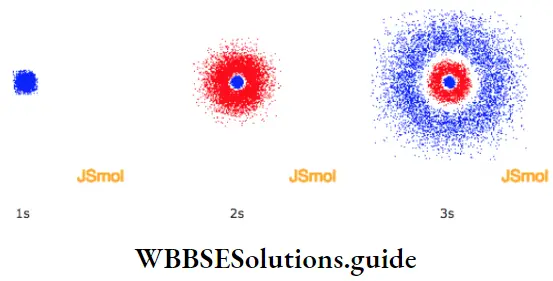
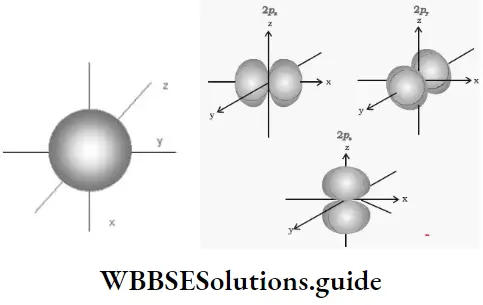
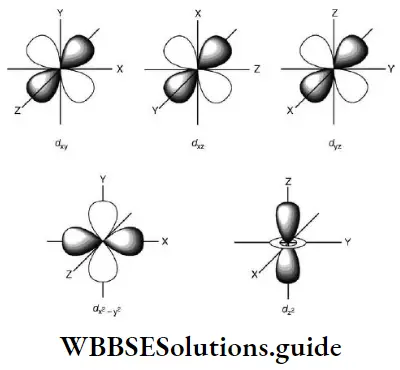

 2. As long as an electron revolves in a particular orbit or shell, it neither absorbs, nor emits energy. In other words, the energy of an electron is constant in a particular orbit. This is why an orbit is referred to as a stationary state. It is a stationary state in terms of energy.
2. As long as an electron revolves in a particular orbit or shell, it neither absorbs, nor emits energy. In other words, the energy of an electron is constant in a particular orbit. This is why an orbit is referred to as a stationary state. It is a stationary state in terms of energy.