Organic Chemistry—Some Basic Principles And Techniques
The famous Swedish chemist, Berzelius, proposed in 1807 that chemicals should be divided into two separate groups—organic and inorganic.
It was believed that organic compounds were those that existed in or were created by living organisms by some ‘vital force’. And that inorganic compounds were simple and could be obtained from minerals.
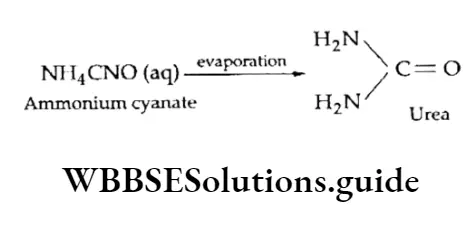
However, this concept became outmoded when Wohler prepared urea, an organic compound, from an inorganic compound— ammonium cyanate.
The compound (urea) prepared was identical to the one found in urine.
This was followed by the synthesis and analysis of many more organic compounds. The modem definition of organic compounds states that such compounds are those that contain carbon.
However, carbon dioxide, carbon monoxide and carbon disulphide are considered to be inorganic compounds.
| Class 11 Biology | Class 11 Chemistry |
| Class 11 Chemistry | Class 11 Physics |
| Class 11 Biology MCQs | Class 11 Physics MCQs |
| Class 11 Biology | Class 11 Physics Notes |
It is now well known that most organic compounds contain, apart from carbon and hydrogen, other elements like oxygen, nitrogen, sulphur, phosphorus and halogens.
Given this, organic chemistry is now regarded as the chemistry of hydrocarbons (compounds containing carbon and hydrogen only) and their derivatives.
“Organic Chemistry, Some Basic Principles and Techniques, for beginners”
It is interesting to know that the total number of organic compounds is much more than that of the compounds of all other elements taken together.
What is so specific about carbon that it forms so many compounds? A unique property to form bonds with other carbon atoms.
This property is referred to as catenation. Owing to this characteristic property, carbon can form straight-chain compounds, branched-chain compounds and rings of different sizes.

Structure Of Organic Compounds
As you already know, carbon being tetravalent, methane has four covalent bonds, one each between a single carbon atom and each of the four hydrogen atoms.
You have already about hybridisation, i.e., the intermixing of atomic orbitals to form new orbitals.
In the case of methane, the three 2p orbitals of the carbon atom intermix with the 2s orbital to produce four sp3 hybrid orbitals which form four covalent bonds with the Is orbital of four hydrogen atoms.
The structure of ethene and ethyne molecules which have been discussed while we studied sp2 and sp hybridisation respectively. To understand and predict the properties of organic compounds, it is important to know their molecular structures. sp2
The nature of orbitals depends on the type of hybridisation they have resulted from.
Nature of orbitals
In sp3 hybridisation, the four sp3 hybrid orbitals of carbon are formed by mixing one 2s and three 2p orbitals.
So each sp3 orbital has 25% s and 75% p character. The percentage changes in sp2 hybridisation, to 33% s and 66% p character.
The sp hybridised carbon has orbitals with 50% s and 50% p character. With more s character the carbon atom becomes more electronegative in comparison to other carbon atoms (sp2 and sp3 hybridised carbon atoms). This is because of the closeness of the s orbital to the nucleus.
Relative bond length and strength of hybrid orbitals
The bond length and the strength of the bonds formed by the combining of hybrid orbitals are influenced by the type of hybridisation in compounds. You know that hybridisation is a theoretical concept.
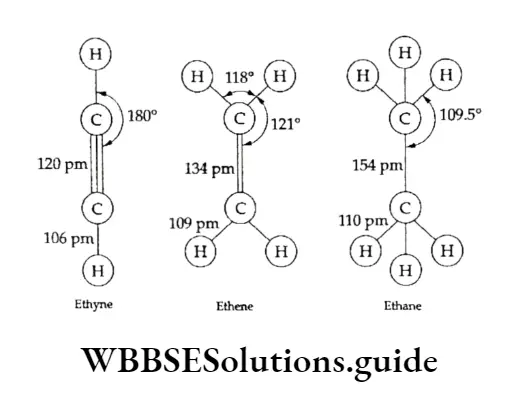
Therefore, the energy of hybrid orbitals is theoretically estimated. Let us see how bond length and strength are influenced by hybridisation considering the examples of ethane, ethene and ethyne.
The sp hybrid orbital of carbon in ethyne is shorter and stronger than the sp2 hybrid orbital in ethene, owing to the 50% s character in ethyne, more than that in ethene.
The sp3 hybrid orbital of carbon in ethane forms longer and weaker bonds than those in ethene and ethyne. This can be attributed to less s character (25%) in the sp hybrid orbital. The distribution of hybrid orbitals in space leads to different bond angles.
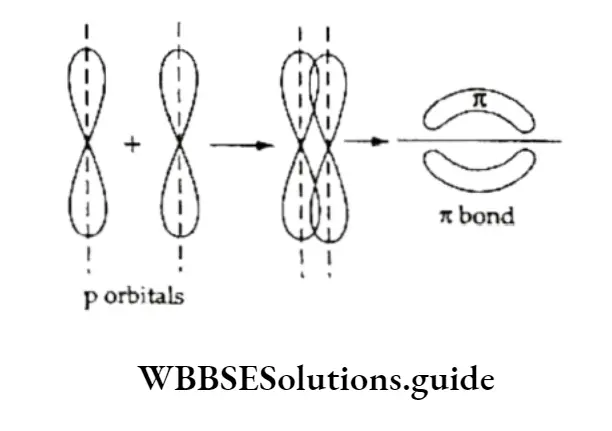
The π Bond
A double bond exists between two carbon atoms in a molecule of ethene. Out of two, one is a bond (formed by the overlap of sp2 hybrid orbitals, one each from each of the carbon atoms) and the other is it bond (formed by the overlap of the two 2pz unhybridised orbitals of each carbon atom).
Similarly, the triple bond in a molecule of ethyne comprises one a bond and two n bonds. You have already studied the two types of bonds. Let us discuss some characteristic features of JI bonds.
The pi (it) bond is formed by the overlap of two 2pz unhybridised orbitals. The overlapping (sidewise or lateral overlap) takes place in such a way that their axes remain parallel to each other and perpendicular to the internuclear axis.
The n bond is weaker than the bond. Due to lateral overlap, the electron charge cloud of the n bond is placed above and below the intermolecular axis.
This makes it easier for the reagent to attack n electrons than electrons. Acetylene, which has a triple bond between the carbon atoms, contains one a and two its bonds, and is, therefore, readily attacked by oxidising reagents.
Structural Representation Of An Organic Molecule
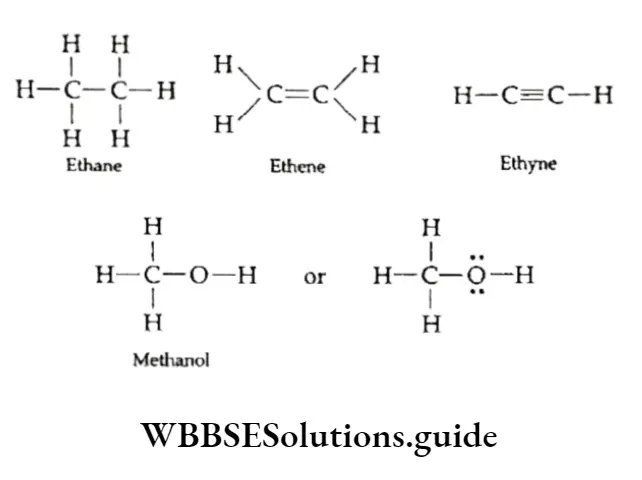
The structures of organic molecules can be represented in a number of ways. The simplest way of representing the structure of an organic compound is by representing a bond by a dash, viz., a single bond by a single dash (—), a double bond by two dashes (=) and a triple bond by three dashes (=).
The lone pair of electrons on atoms like oxygen, nitrogen and sulphur may or may not be shown. Some examples of such complete structural formulas are given below.
Organic molecules can also be represented by condensed structural formulae. In this type of representation, some or all of the covalent bonds in the molecule are omitted and the number of each of the identical atoms is indicated by a subscript.
⇒ \(\begin{array}{ccc}
\mathrm{CH}_3 \mathrm{CH}_3 & \mathrm{H}_2 \mathrm{C}=\mathrm{CH}_2 & \mathrm{HC} \equiv \mathrm{CH} \\
\text { or } & \text { or } & \text { or } \\
\mathrm{C}_2 \mathrm{H}_6 & \mathrm{C}_2 \mathrm{H}_4 & \mathrm{C}_2 \mathrm{H}_2 \\
\text { Ethane } & \text { Ethene } & \text { Ethyne }
\end{array}\)
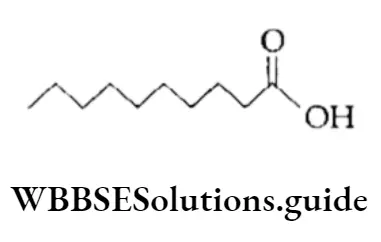
Similarly, CH3CH2CH2CH22CH2CH2CH2CH2CH2COOH can be condensed to CH3 (CH2)8COOH. In bond line representation only bonds or lines are used to represent a molecule, and carbon and hydrogen atoms are not shown.
The end of each line represents a carbon atom which is understood to be attached to the required number of hydrogen atoms necessary to satisfy the tetravalency of carbon.
The lines are drawn in a zig-zag fashion and represent carbon-carbon bonds. The atoms nitrogen, oxygen and chlorine are specifically written.
The terminals denote the methyl (—CH3) group unless otherwise indicated. Thus CH3CH2CH2CH2CH2CH2CH2CH2CH2COOH can be represented as
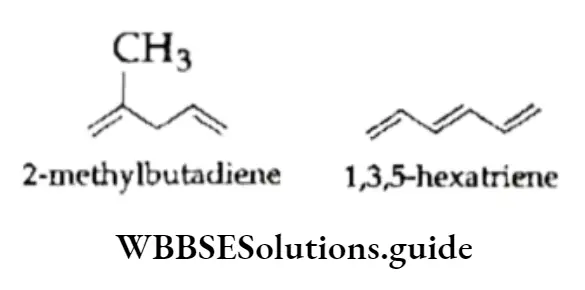
In the same way, the bond line formulas of 2-methyl butadiene and 1,3,5-hexatriene are written as follows
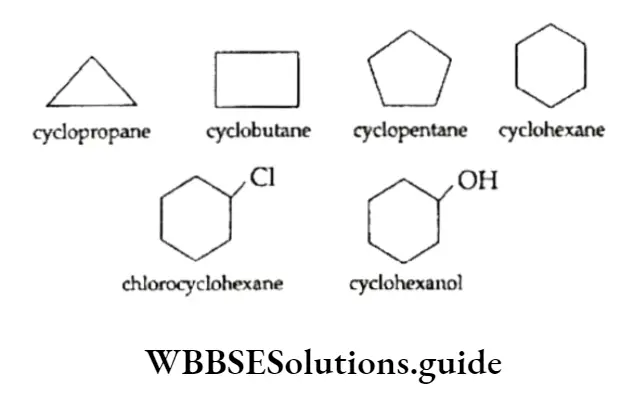
A compound containing one or more rings is called a cyclic compound and is represented by drawing the appropriate ring(s) without showing C and H atoms.
The comers of the ring represent C atoms and the sides I denote carbon-carbon bonds. Any functional group present is also shown in the structure.
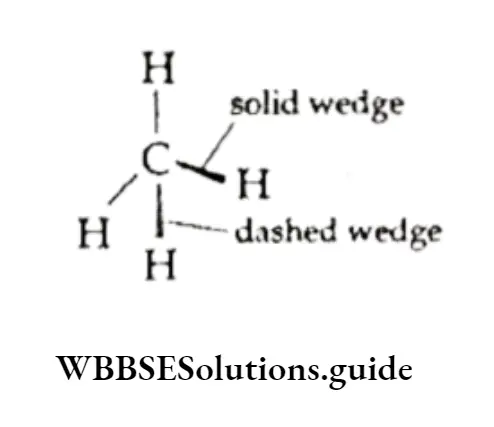
The wedge-and-dash representation is used to represent the three-dimensional structure of a molecule.
By convention, a solid wedge indicates a bond projecting from the plane of paper towards the viewer and a dashed wedge shows a bond projecting inwards from the plane of the paper.
A normal line indicates the bond on the plane of the paper. The three-dimensional representation of methane is shown here.
Functional Groups
A functional group is an atom or n group of atoms responsible for the chemical behaviour of an organic compound. Structural features like double and triple bonds are also considered to be functional groups.
In an organic molecule, the functional group is the most reactive part whereas the remaining portion determines the physical properties of the compound. For example, amine).
If we attach the — NH2 (amino) as a functional group In CH3CH2NH2 (ethyl —NH2 group to some other alkyl radical like CH-,—(methyl) or I7 —(propyl), we would get the compounds CH3 NH2 and C3H7NH2.
Compounds containing the same functional group undergo more or less similar reactions and are, therefore, said to belong to n family. There may be exceptions in the case of very large molecules or if more than one functional group is present.
Hie compounds we have just discussed belong to a family called amines. Some other functional groups which you may come across at the present level of learning.
Homologous Series
A family of organic compounds like alkanes and alcohols contains a characteristic functional group and forms what is known as a homologous series.
Each member of the series is known as a homologue. The members differ from each other in molecular formulae by a —CH2 unit.
The first four members of the homologous series of the alcohol family are as follows.
⇒ \(\begin{array}{ll}
\mathrm{CH}_3 \mathrm{OH} & \text { Methyl alcohol } \\
\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{OH} & \text { Ethyl alcohol } \\
\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{OH} & \text { Propyl alcohol } \\
\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{OH} & \text { Butyl alcohol }
\end{array}\)
Characteristics of a homologous series
- A particular homologous series can be represented by a general formula. For example, in the case of alcohols, the general molecular formula is CnH2n+1OH. By putting the value of n as 1, 2, 3, 4,… where n is the number of carbon atoms in the molecule, we get the molecular formula of the corresponding homologues.
- Any member of the homologous series differs from the next lower or higher by the unit CH2(methylene group) or by 14 atomic mass units (12 + 2 x 1 = 14).
- The different homologues of the series can be prepared by employing general methods of preparation.
- The members of a homologous series show similar chemical properties.
- The physical properties (such as density, melting point, and boiling point) of the successive members in a homologous series show a more or less regular gradation.
Nomenclature Of Organic Compounds
Hydrocarbons are compounds which contain only hydrogen and carbon. They are the simplest organic compounds.
Organic compounds bearing functional group(s) are derived from hydrocarbons by replacing one or more hydrogen atoms with the functional group.
As more and more carbon compounds were known it became necessary to introduce a system of nomenclature.
A formal system of nomenclature of organic compounds was introduced in 1931, known as the IUC system.
The system was further developed by the International Union of Pure and Applied Chemistry (IUPAC).
Before the IUPAC system of nomenclature was introduced, organic compounds were given common or trivial names. The names were based on the source of the compounds or some other specific features.
“Organic Chemistry, Some Basic Principles and Techniques, notes PDF”
For example, acetic add can be obtained from vinegar and was, therefore, named after the Latin word for vinegar, aceluin. Some ants contain formic acid and the Latin word for ant isformica.
Urea was so named since it was first isolated from the urine of mammals. Methyl alcohol was called wood spirit since it could be obtained by the destructive distillation of wood.
Many of the common names are still used by chemists. Common names are useful when the other systematic names are very complicated.
The newly discovered allotrope of carbon (C60) has been given the common name Buckminsterfullerene, after the architect Buckminster Fuller, whose designs are similar in structure to the C60 cluster of carbon.
The IUPAC System Of Nomenclature
The IUPAC system is simple, and any chemist can deduce the structure of an organic compound if he or she knows its IUPAC name. In the IUPAC system of nomenclature, a chemical name has three parts: prefix, parent and suffix The parent name tells us how many carbon atoms are present in the principal chain.
The suffixes are of two types—primary and secondary, and both indicate the functional groups present in the compound.
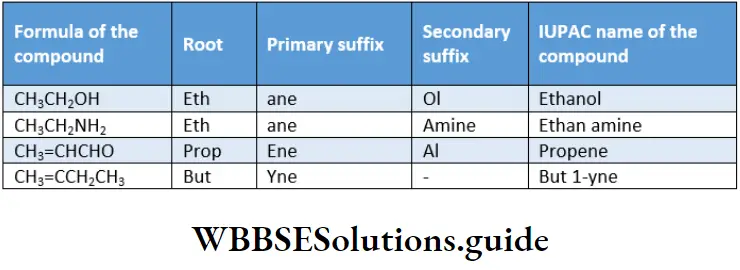
The primary suffix is added to the root derived from the parent name indicating the nature of the bond between the carbon atoms. For example, the suffix one in ethyne shows the presence of a triple bond.
The secondary suffix is added after the primary suffix to indicate the functional group(s) present in the carbon chain. For example, the IUPAC name of alcohol—alkanol—has two suffixes an and ol.
Here an indicates a single C —C bond and ol indicates the class of the compound—alcohol.
Note While adding the secondary suffix to the primary suffix, the terminal e of the primary suffix is removed if the secondary suffix begins with a vowel but it is retained if the secondary suffix begins with a consonant.
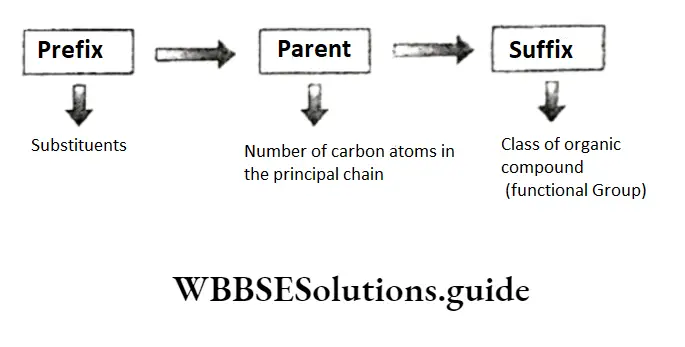
The prefixes indicating substituents on the parent chain are also of two types—primary and secondary.
A primary prefix such as ‘cyclo’ is added before the root word to indicate the cyclic nature of the carbon skeleton. For example, cyclohexane.
In a polyfunctional compound the groups which are not considered the principal functional group in the IUPAC system of nomenclature, but regarded as substituents, are called secondary prefixes.
For example, halogens, alkoxy, alkyl, aryl and nitro groups. The secondary prefix comes before the primary prefix. For example,

To begin with, let us study the systemic IUPAC nomenclature of hydrocarbons. On the basis of their structure, hydrocarbons may be of three types—straight-chain, branched-chain and cyclic.

Alkanes
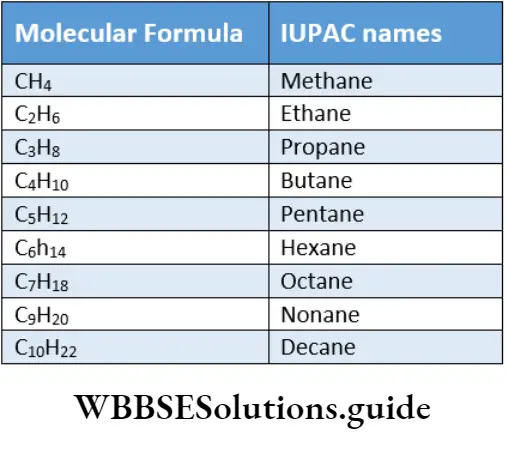
Primary prefix Secondary prefix Root word Primary suffix IUPAC name cydo Chloro hex ane Chlorocyclohexane The root word for alkanes from CH4 to C4H10 are derived from trivial names.
However, the names of alkanes containing five or more carbon atoms are derived by adding prefixes such as pent (five), hex (six), hept (seven), oct (eight) and so on, indicating the number of carbon atoms in the molecule, with the suffix ane. The molecular formula and the IUPAC names of some straight-chain alkanes.
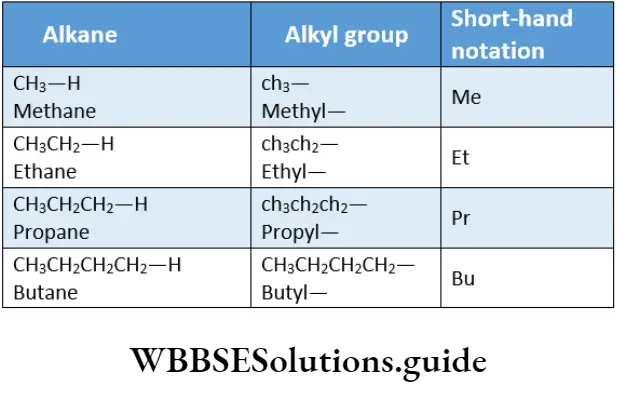
On removing one hydrogen atom from an alkane, a univalent alkyl group is formed. The individual alkyl group is named by replacing the suffix ane of the parent hydrocarbon with yl.
Branched-chain alkanes are named according to the following rules.
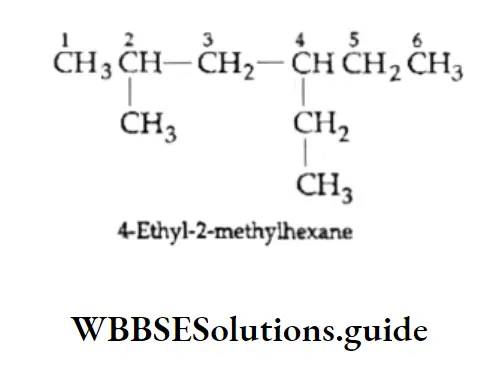
1. The longest continuous chain of carbon atoms is the parent chain. For example, the following compound is a hexane since the longest chain contains six carbon atoms.
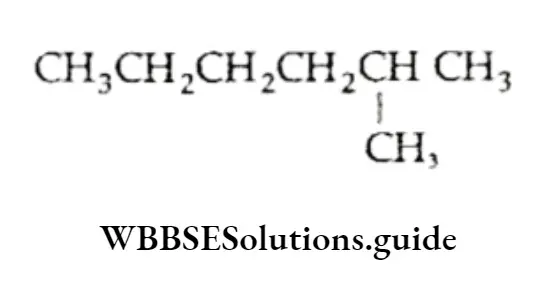
The following compound is a heptane since the longest chain has seven carbon atoms.
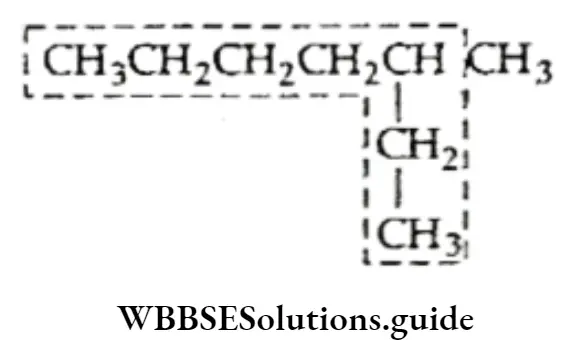
In case a molecule contains two equally long carbon chains, the one attached to a greater number of side chains or substituents is selected. Correct longest chain: Parent chain of seven carbon atoms with one side chain and two substituents.
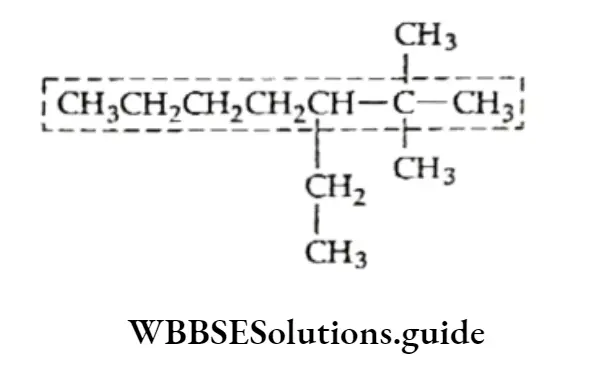
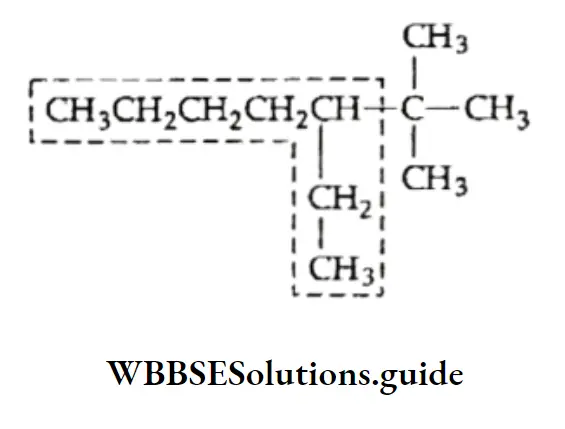
Incorrect longest chain: Parent chain of seven carbon atoms with only one side drain.
2. For the longest chain from one end to the other, the direction of numbering is chosen to give the lower number(s) to the substituent(s).
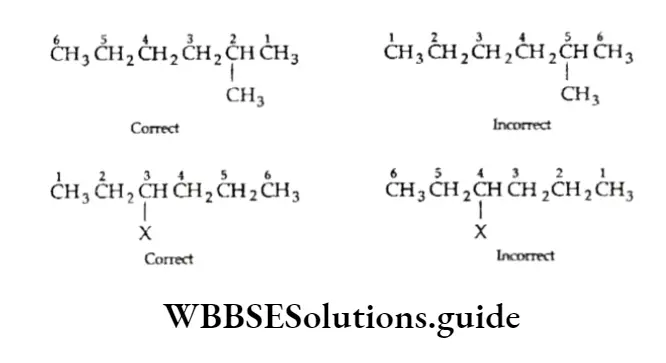
3. The location of the substituent groups is designated by using rule 2. The number indicating the position of the substituent should be prefixed to the name of the parent alkane.
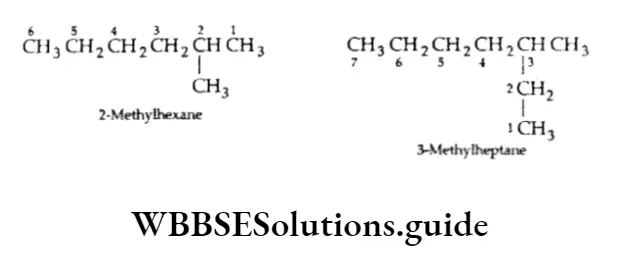
The numbers are separated from the groups by hyphens. Note that the following common names are retained by IUPAC for unsubstituted hydrocarbons only.
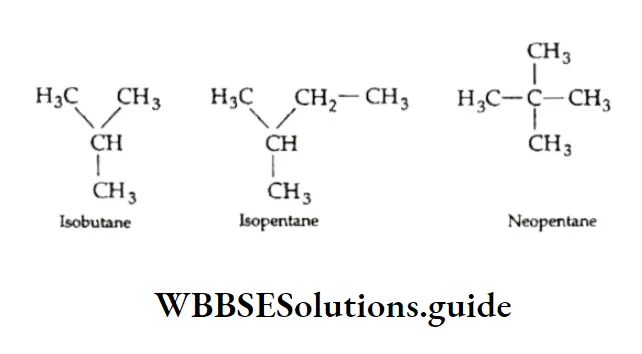
Precisely speaking, isobutane is 2-methylpropane, isopentane is 2-methylbutane and neopentane is 2, 2-dimethylpropane.
Lowest sum rule: In case the parent chain has two or more side chains or substituents, the numbering must be done in such a way that the sum of the locants on the parent chain is the lowest possible.
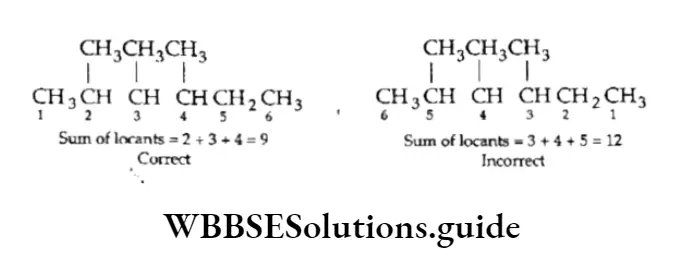
4. When different substituents are present on the carbon chain, they are written in alphabetical order.
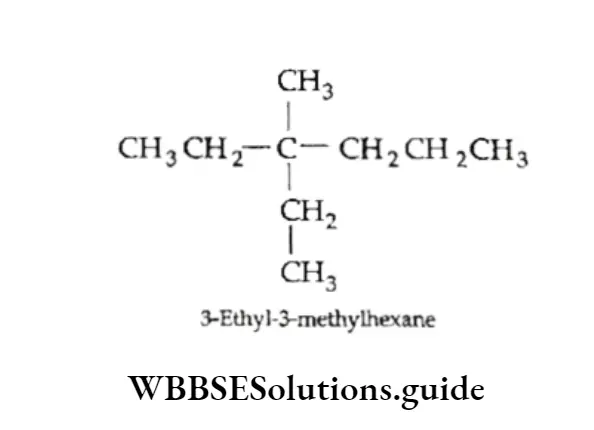
5. In case two substituents are present on the same carbon atom, the number indicating the position is written twice.
6. Identical substituents are indicated by using appropriate prefixes di, tri, tetra, etc. The numbers are separated by commas.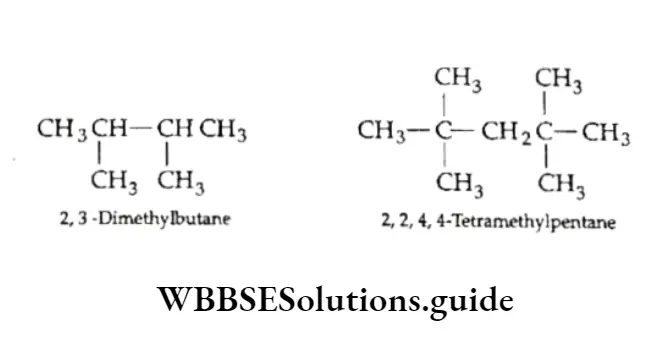
When two chains of equal length compete for selection as the parent chain, choose the chain with the greater number of substituents.
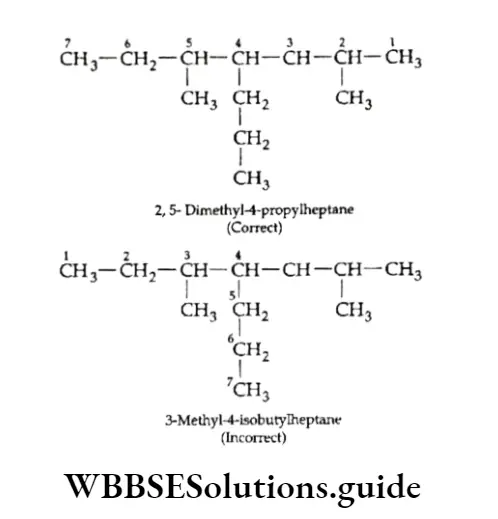
When two different substituents are in equivalent positions (from the two ends of the carbon chain), the groups are numbered in alphabetical order, while numbering the chain.
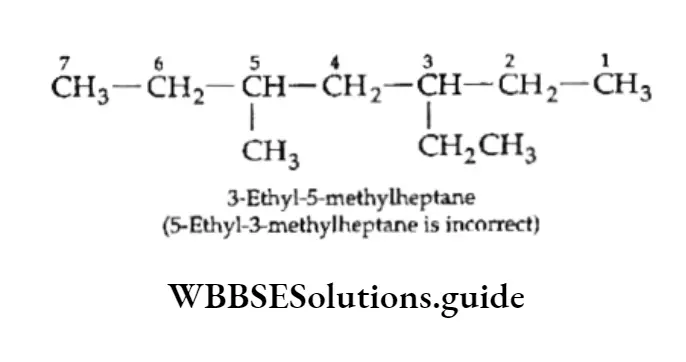
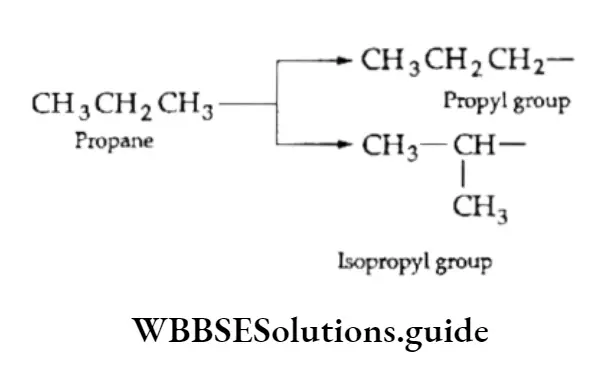
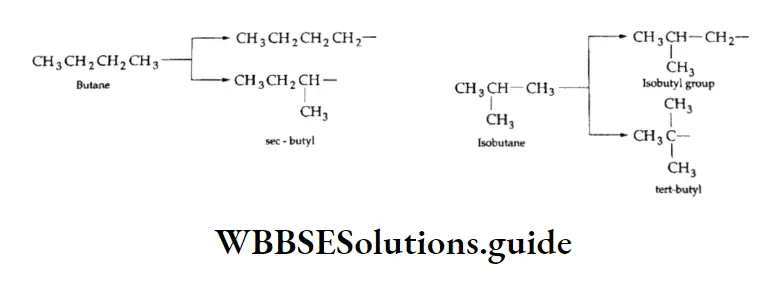
On removal of one hydrogen atom, both butane and isobutane give rise to two butyl groups.
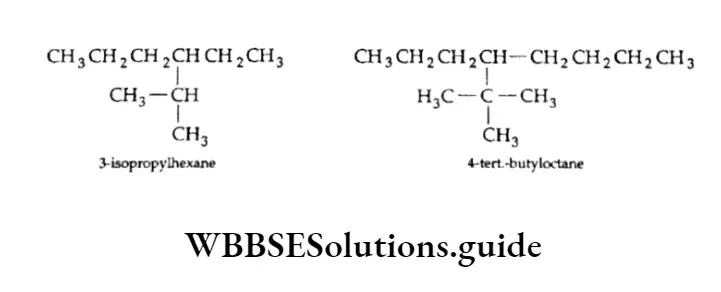
The following examples illustrate the naming of compounds containing branched alkyl groups.
The prefixes iso, see, test and neo are approved by 1UPAC

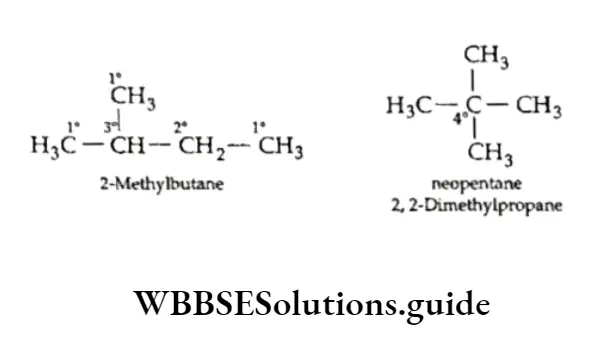
Classification of carbon atoms
Being tetravalent, a carbon atom is attached to four other carbon or hydrogen atoms in hydrocarbons.
One carbon atom may be attached to one other carbon atom and three hydrogen atoms. Also, one carbon atom may be attached to three other carbon atoms and one hydrogen atom.
Depending upon these arrangements carbon atoms are classified into four types atom is attached to one other carbon—the primary atom.(1°), A secondary secondary carbon (2°), tertiary atom is(3°) attached to quaternary two other(4°). carbonA primary atoms, carbon and so on.
A hydrogen atom is also referred to as 1°, 2° or 3° depending on whether it is attached to a 1°, 2° or 3° carbon atom. In the following examples, 1°, 2°, 3° or 4° carbon atoms are shown.
Cycloalkanes
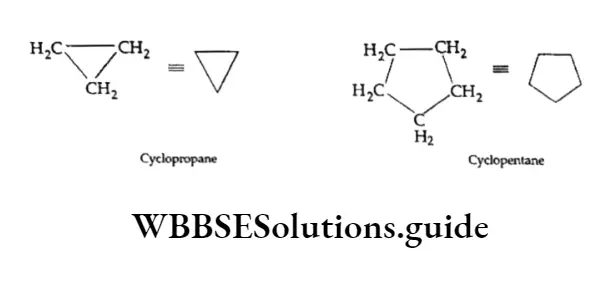
Cycloalkanes with only one ring are named by attaching the prefix ‘cydo’ to the names of the corresponding straight-chain alkanes. For example,
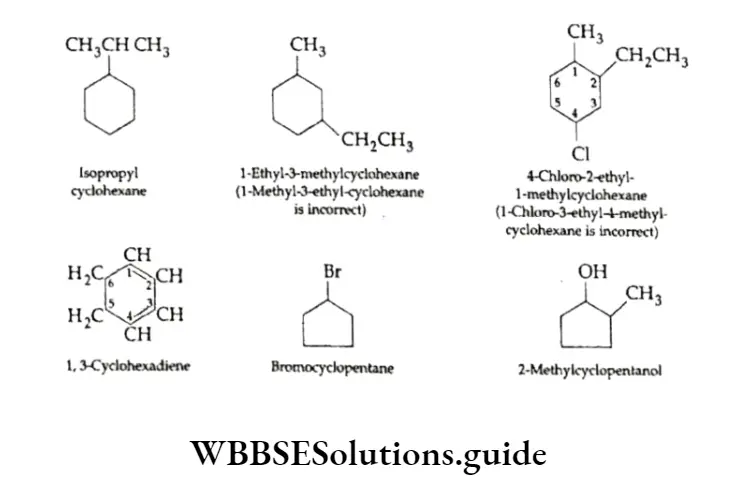
Substituted cycloalkanes are named applying the same rules as applied to branched-chain alkanes. If more than one substituent is present, its position is indicated by the appropriate number.
While naming the compound, the substituents are placed in alphabetical order. Also, the numbering is so done that the substituent(s) get the lowest possible number(s). The following examples illustrate the abovementioned points.
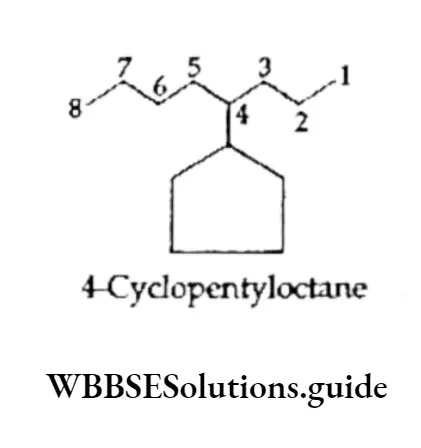
When a single ring is attached to a single chain with the same or a greater number of carbon atoms than in the ring, the compound is named as follows.
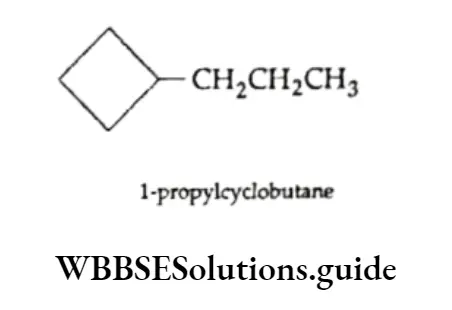
The carbon atom attached to the more-branched chain is assigned the lower number.
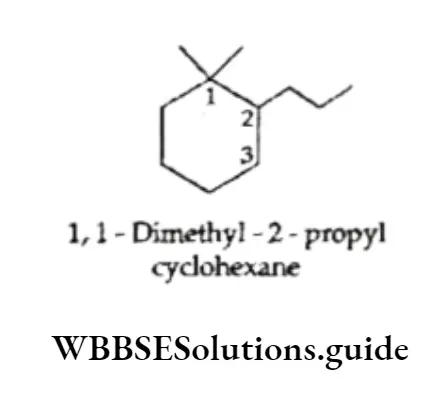
When the chain attached to the ring is longer than the ring, the ring becomes a substituent.
Alkenes
Alkenes are unsaturated hydrocarbons containing a double bond between two adjacent carbon atoms.
Ethene or ethylene is the first member of this homologous series. The rules for the IUPAC nomenclature of alkenes are similar in many respects to those of alkanes.
1. The longest carbon chain containing the double bond is chosen as the parent alkene. The suffix part of the corresponding alkane is changed into one. For example, the carbon chain with six carbon atoms and a double bond is hexene.
“Organic Chemistry, Some Basic Principles and Techniques, study material”
2. The numbering of the carbon chain is so done that the double bond has the lowest number. Designate the location of the double bond by writing the lower number as a prefix. The locant (number) is placed just before the functional group suffix.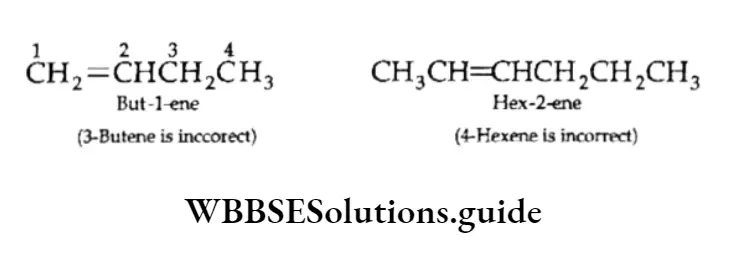
3. The locations of the different substituents are indicated by the numbers of the carbon atoms to which they are attached. The lowest sum rule studied earlier in the case of alkanes also applies here.
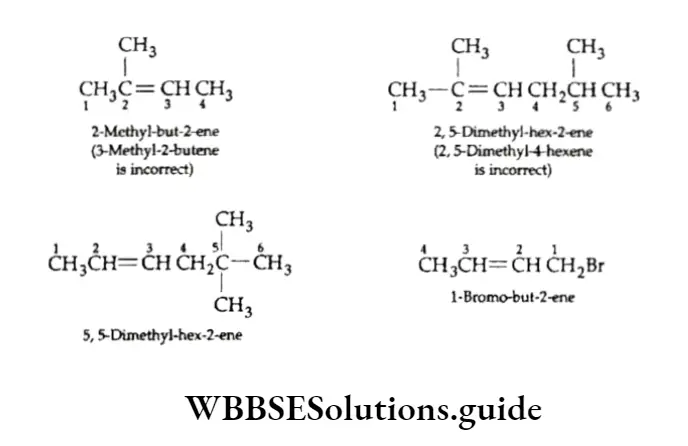
4. Cycloalkenes are numbered in a way that gives the carbon atoms of the double bond the 1- and 2- positions and that also gives the substituent groups the lower numbers.
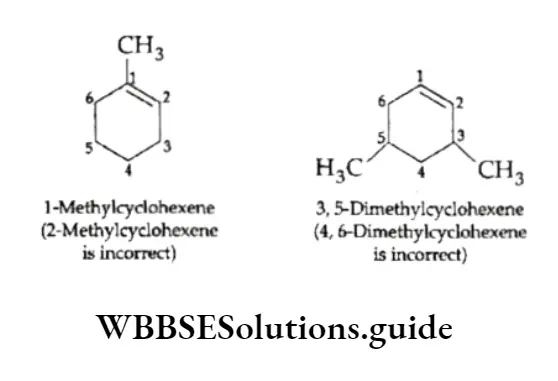
5. Compounds containing a double bond and an alcohol group are named by giving the alcohol carbon the lowest number.
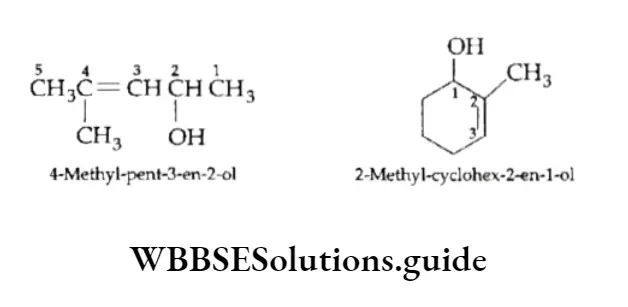
The terminal e in the name of the alkene is dropped if it is followed by a suffix beginning with a, i,o, u or y. (The rule is the same for alkynes.)
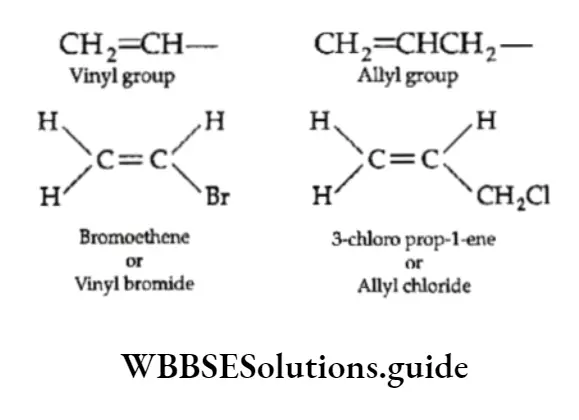
6. The removal of the terminal hydrogen atom from ethene and propene gives the vinyl and the allyl group respectively. If a halogen atom is attached to these groups we obtain compounds with common names vinyl halide and allyl halide.
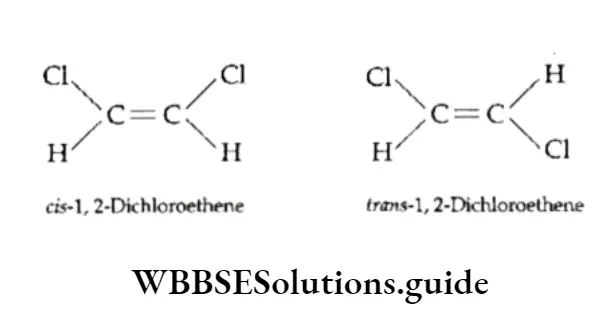
7. If two identical groups are on the same side of the double bond, the compound is designated as cis and if they are on opposite sides, it is designated as trans.
8. If there are two or three double bonds in a chain then the compound is called a diene or a triene respectively.
⇒ \(
\stackrel{1}{\mathrm{C}} \mathrm{H}_2=\stackrel{2}{\mathrm{C}} \mathrm{H}-\stackrel{3}{\mathrm{C}} \mathrm{H}=\stackrel{4}{\mathrm{C}} \mathrm{H}_2
Buta-1,3-diene\)
Alkynes
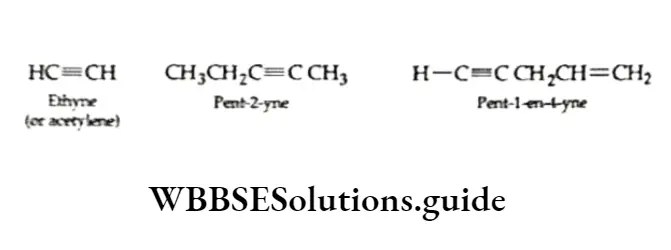
Alkynes are unsaturated hydrocarbons that contain a triple bond between two adjacent carbon atoms. The first member of the homologous series is acetylene or ethyne (HC=CH)
Alkynes are named in the same way as alkenes. The unbranched alkynes are named by replacing the suffix ane of the corresponding alkane with the suffix one. The first three members of the alkyne homologous scries ethyne, propyne and butyner.
The trivial name acetylene has been retained by the IUPAC system for HC=CH and is frequently used.
Where the double as well as the triple bond is present in the same molecule, the double bond is given the lowest number (order of priority of functional groups).
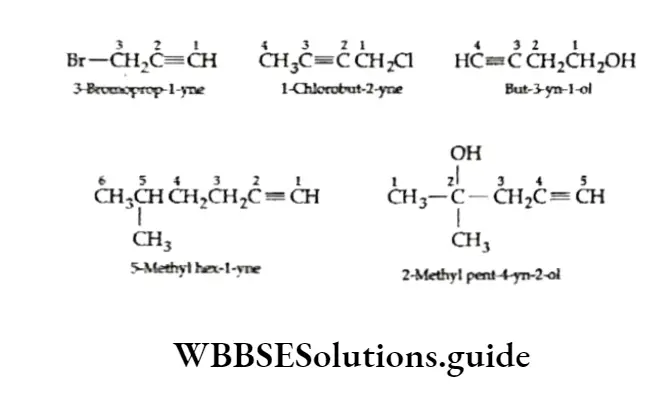
The locations of substituent groups of substituted alkynes are also indicated with the appropriate numbers. A hydroxyl (—OH) group is given priority over the triple bond while numbering the chain.
Here you should remember that the terminal e of meor yne is not dropped in case it is followed by di, tri or tetra.
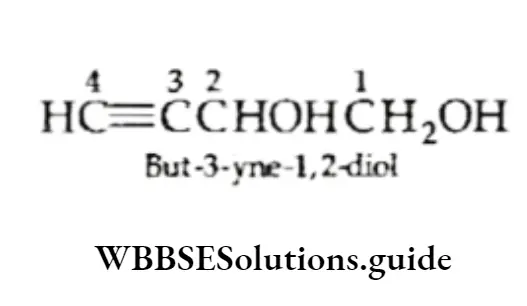
Polyfunctional compounds
As stated earlier in the chapter, compounds containing the same functional group belong to a family. While naming the compound, the functional group is identified and the appropriate suffix is used.
The parent chain is so numbered that the carbon atom attached to the functional group gets the lowest possible number (as in the case of alkenes and alkynes).
But what if the compound has more than one functional group? In the case of polyfunctional compounds, one of the functional groups is chosen as the principal functional group according to the order of priority of functional groups.
The compound is named on the basis of that principal functional group and the remaining functional group(s) is named as substituents using appropriate prefixes.
The order of priority of some functional groups is COOH, —S03H, —COOR, —COG, —CONH2, —CN, =C—. —HC=0, —OH, -NH2, /C=CC
The alkyl (—R), phenyl (—C6H5), halogens (F, Cl, Br, I), nitro (—NOz), alkoxy (—OR), etc., are substituents and always prefixed.
Along with the different families of compounds, their respective prefixes and suffixes, Table 12.4 also contains examples of polyfunctional compounds and their IUPAC names.
Example Name the following compounds according to IUPAC rules
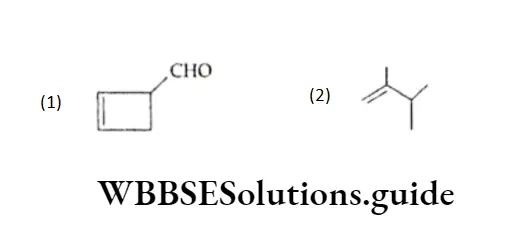
Solution:
- The longest continuous chain is cyclic and of four carbon atoms. Hence the corresponding hydrocarbon is cyclobutane.
- Two functional groups (a double bond and an aldehyde) are present in the compound.
- According to the order of priority of functional groups, the aldehyde precedes the alkene.
- Hence the carbon attached to —CHO is numbered 1.
- Hence, the name of the compound is l-Formylcyclobut-2-ene.
- The longest continuous chain (with two substituents) is of four carbon atoms, so the root word is but.
- Applying the lowest sum rule, the two substituents methyl (—CH3) are at carbons 2 and 3. The double bond is at position 1 of the carbon chain.
- Hence, the name of the compound is 2,3-Dimethyl-but-l-ene.
Aromatic Compounds
Benzene and substituted benzene compounds are the most important members of this class. Benzene contains six carbon atoms, numbered 1 to 6. The molecular formula of benzene is C6H6. It contains three double bonds.
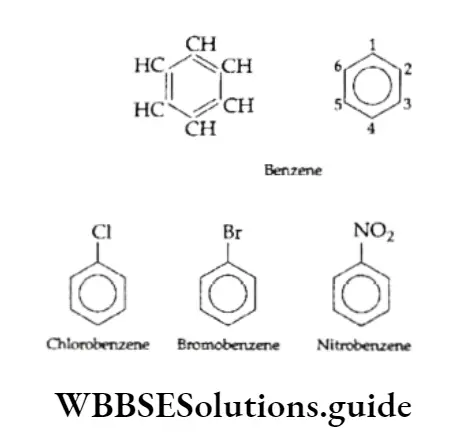
The six hydrogen atoms in benzene are equivalent, each bonded to one carbon atom. Owing to this only one monosubstituted derivative is possible. But benzene can form three disubstituted derivatives with relative positions; 1,2;13 and 1,4.
The positions are indicated by trivial terms, ortho (o), meta (m) and para (p) respectively. While naming these derivatives according to IUPAC rules, we simply prefix the name of the substituent group to the word benzene.
Some derivatives of benzene have universally accepted, common names (written in brackets below) which have no resemblance to the names of the attached substituent.
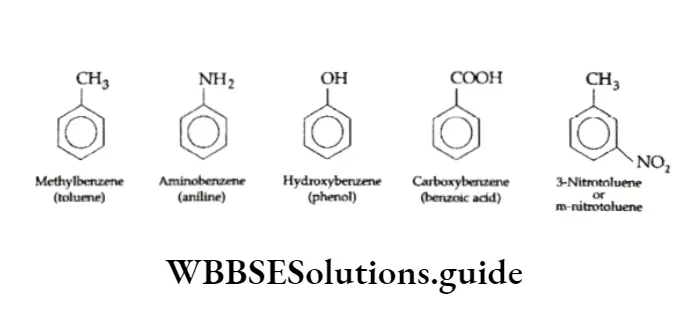
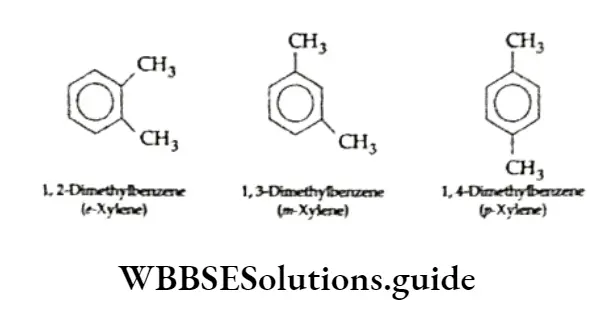
Disubstituted or trisubstituted benzenes are named by numbering the positions of the substituents.
Dimethylbenzenes arc commonly called xylenes.
If different substituents are attached to the benzene ring then the following rules are observed.
- The principal group or substituent of the base compound is assigned the number 1.
- The numbering is so done that the substituents are located at the lowest numbers possible.
- The substituents are written in alphabetical order.
- In some cases, the common name of the die benzene derivative is considered the name of the parent compound.
Let us now look at a few categories of benzene derivatives.
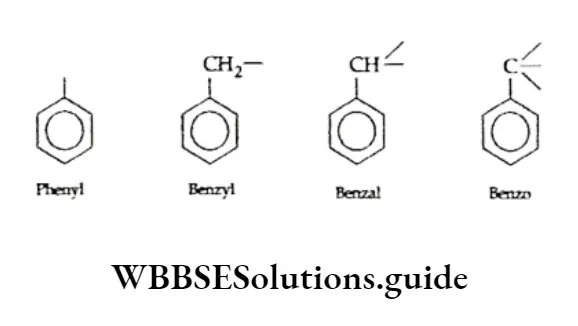
The trivial names phenyl, benzyl, benzal and benzo have been accepted by IUPAC
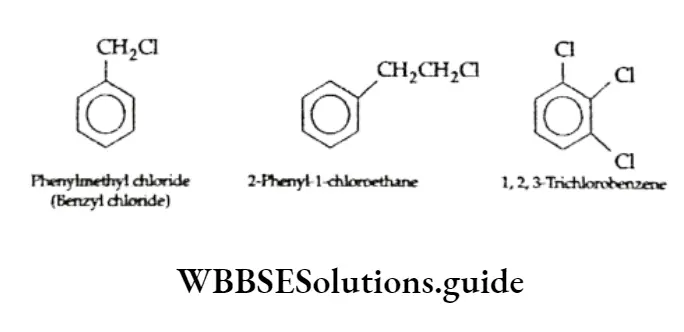
Halogen derivatives.
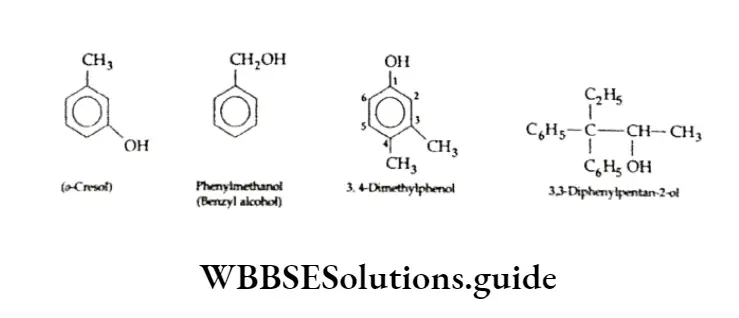
Hydroxy derivatives
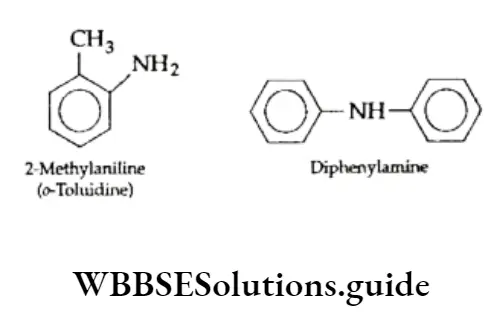
Amines
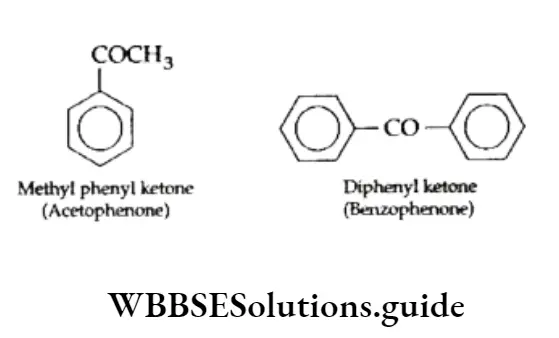
Ketones
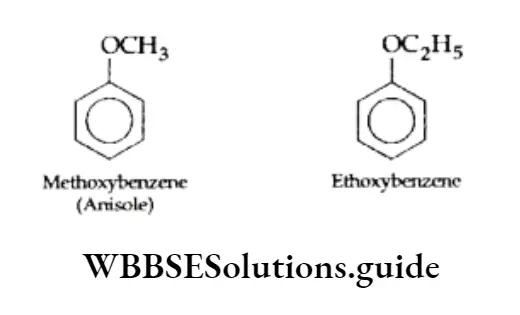
Aromatic ethers
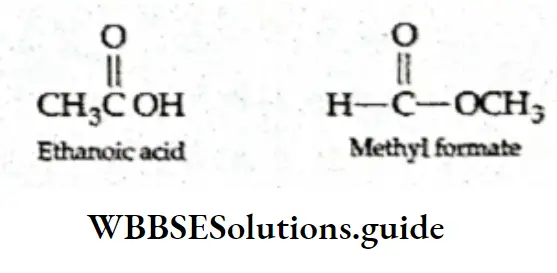
Aldehydes
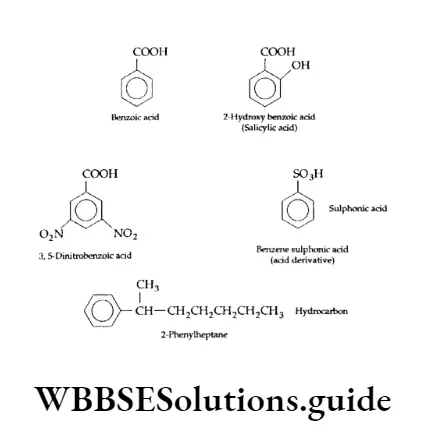
Carboxylic Acids
Isomerism
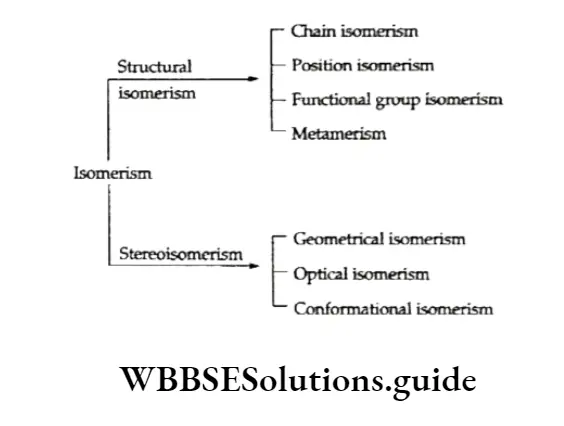
When two or more compounds have the same molecular formula but differ in their physical and chemical properties, the phenomenon is called isomerism, and the compounds isomers (Greek, isos = equal; meros = parts).
Isomerism is of two types—structural isomerism and stereoisomerism.
Structural isomers are those which differ in the mode of linking of atoms whereas stereoisomers differ in the arrangement of atoms or groups in space. Stereoisomerism.
Structural isomerism is of different types, based on different functional groups, different carbon skeletons and different positions of the functional groups in the same carbon chain.
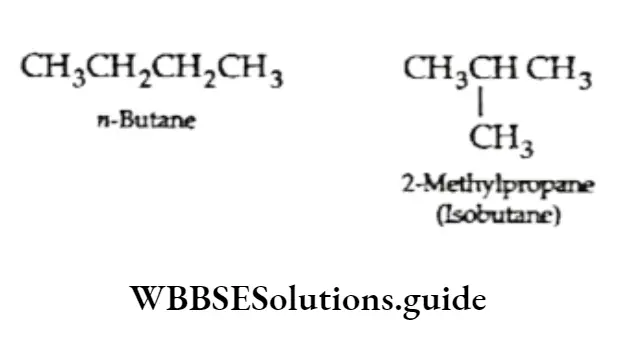
Chain isomerism This type of isomerism arises when the carbon skeletons of the isomers differ. For example, butane (C4H10) can have the following two structures.
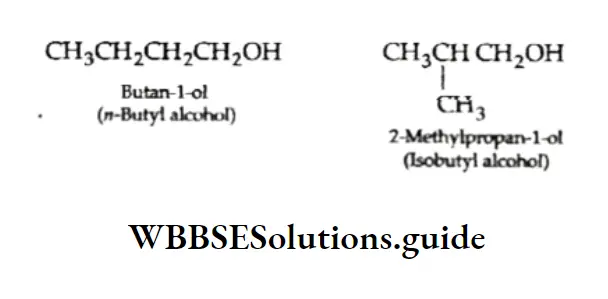
In the same way the alcohol with the molecular formula, C4H9OH can be either n-butyl alcohol or is obutyl alcohol.
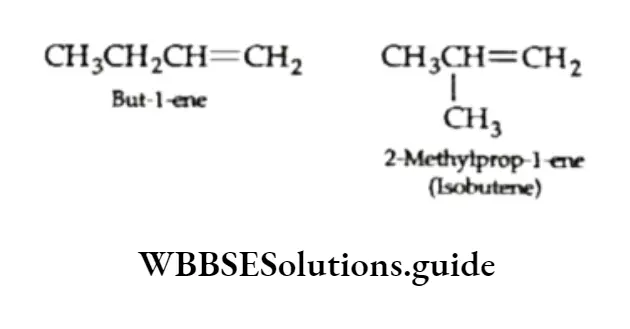
The alkene C4H8 can be either but -1-ene or isobutene.
Position isomerism When the isomers differ in the position of the substituent atom or group in the same carbon chain, the phenomenon is called position isomerism.
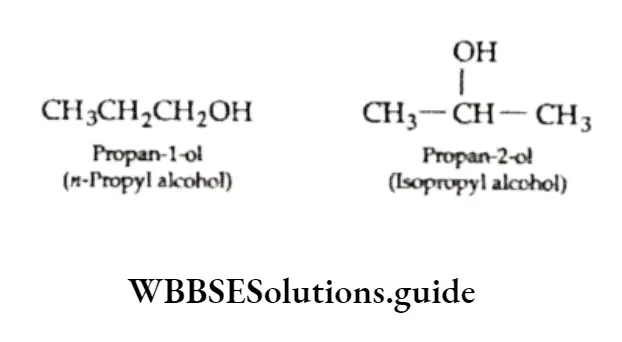
As you can see. the position of the —OH group is different in the same carbon chain of the isomers with the molecular formula C3H7OH.
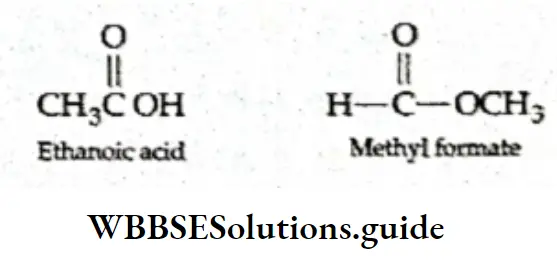
Functional group isomerism When two or more compounds have the same molecular formula but different functional groups, they are called functional group isomers and the phenomenon is called functional group isomerism.
A compound with the molecular formula C4H10 can be an ether or an alcohol.

Similarly, C3H60 can be a ketone or an aldehyde.

C2H40 can be an add or an ester.
Metamerism When the isomers differ in the alkyl groups attached to the same functional group, they are called metamers and the phenomenon is called metamerism.
An ether, C4H10, can be either methyl propyl ether or diethyl ether.
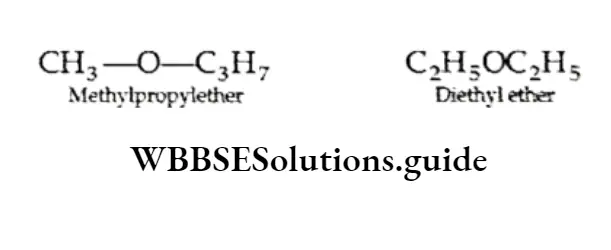
A ketone, C5H10, can have two metamers.
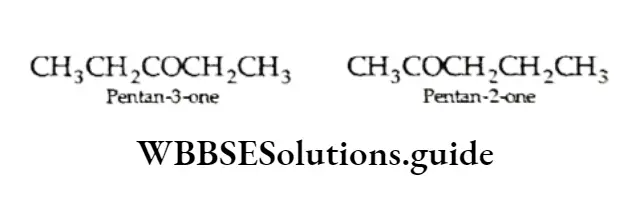
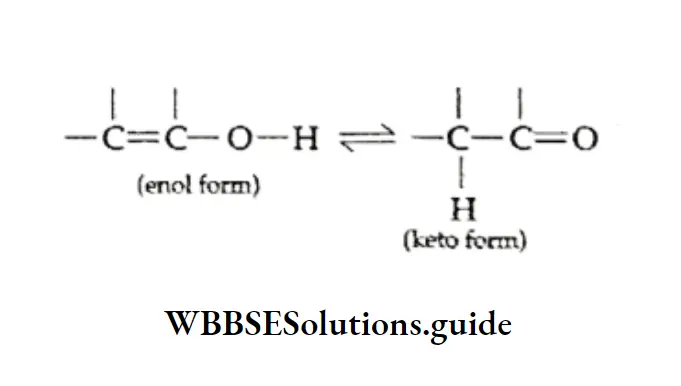
Tautomerism This is a type of isomerism in which the two isomers are in equilibrium. The most common land is keto-enol tautomerism.
In this form of tautomerism compound containing a —CH2—CO— group (the keto form of the molecule) is in equilibrium with one containing the —CH=C(OH)— group (the enol).
This happens when a hydrogen atom migrates from a carbon atom to an oxygen atom bonded to an adjacent carbon.
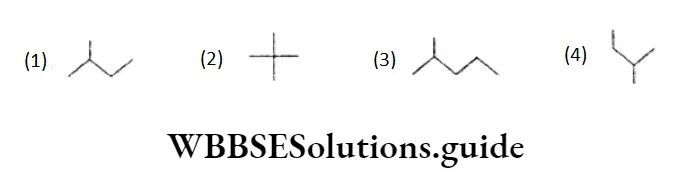
Example What is the relationship between the following molecules and CH3CH(CH3)CH, CH3?
Solution:
- The bond line formula for CH3CH(CH3 )CH2CH3 is which is the same as and Thus, all three molecules are the same.
- Molecule
- Is neopentane, i.e.,
- Molecule is CH3CH(CH3)CH2CH,CH3 which is the next homologue of CH3CH(CH3)CH2CH3.
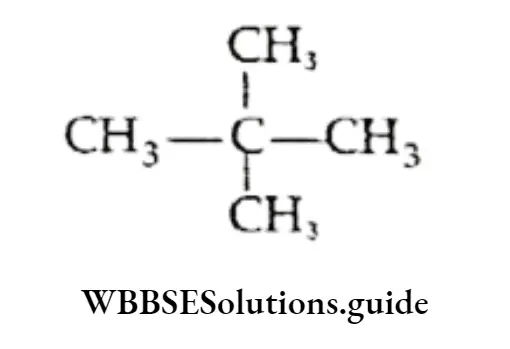
CH3CH(CH3)CH2CH3 and neopentane are chain isomers.
Reaction Mechanisms
Many reactions take place via intermediates, i.e., they are multi-step. When the products in a reaction are formed directly without any intermediate, the reaction is called a single-step reaction. In general, an organic reaction may be represented as follows.
⇒ \(\text { Substrate } \stackrel{\text { reagent }}{\longrightarrow} \text { [Intermediate] } \longrightarrow \text { Products }\)
The steps leading to the cleavage of bonds (covalent bonds in the substrate molecule) and the formation of new bonds in the products constitute the reaction mechanism.
A clear understanding of reaction mechanisms helps us identify patterns in complex organic reactions.
And recognising patterns helps us apply our knowledge about one reaction to many similar reactions.
“Organic Chemistry, Some Basic Principles and Techniques, class 11 NCERT”
The reaction mechanism of a complex organic reaction may depend on many factors such as the following.
- The reactivity of the substrate molecule may be known by an understanding of the electron displacement effects caused due to the interaction of substrate and reagent molecules and the difference in electronegativities of the atoms in a molecule.
- The nature of bond fission decides the type of the reaction intermediate formed. Carbocations, carbanions and free radicals are types of reaction intermediates.
- The nature of the reagent, whether electrophilic or nucleophilic.
- The type of reaction which occurs. Substitution, addition, elimination and rearrangement are some common types of organic reactions. You will study these reactions.
Let us now discuss these factors in greater detail.
Electron Displacement In A Molecule
Generally, unlike inorganic compounds, organic compounds do not react with each other on their own.
A reaction between organic compounds requires the presence of a reagent. Some examples of reagents are H2S04, HC1 and NaOH.
Although a substrate molecule is electrically neutral, it develops some polarity due to the difference in electronegativities of the atoms of the molecule or under the influence of an attacking reagent.
The polarity develops by the displacement of electrons in the substrate. This is referred to as the electron displacement effect.
We normally come across the following four types of such effects in organic molecules.
- Inductive effect
- Electromeric effect
- Mesomeric effect
- Hyperconjugation effect
Inductive effect When a covalent bond is formed between two dissimilar atoms, the shared electron pair will be displaced towards the atom which is more electronegative.
This causes the more electronegative atom to acquire a partial negative charge (5-) whereas a partial positive charge (8+) develops on the other atom.
For example, if we consider the covalent bond C—X, where X is more electronegative than carbon, then the bond is polarised so that C gains a partial positive charge (5+) and X gains a partial negative charge (8-).
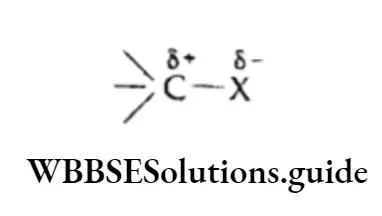
Alternatively, in a covalent bond C—Y, where Y is less electronegative than C, the shared electron pair is displaced towards carbon, so that C attains a 8- charge and y attains a &+ charge.
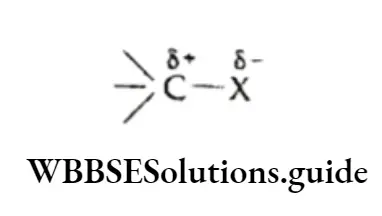
The polarization induced in a substrate molecule is of a permanent nature.
Thus, an inductive effect involves the permanent shift of electrons towards the more electronegative element in a covalent bond.
When the electronegative group linked to a carbon atom withdraws electrons and gains an 8- charge it is said to be an electron-withdrawing group exerting a -I effect.
Some common electron-withdrawing groups (in decreasing order of -I effect) arc:
⇒ \(\mathrm{O}^{-}>\mathrm{CO}_2^{-}>\left(\mathrm{CH}_3\right)_3 \mathrm{C}>\left(\mathrm{CH}_3\right)_2 \mathrm{CH}>\mathrm{CH}_3 \mathrm{CH}_2>\mathrm{CH}_3\)
⇒ \(\mathrm{NO}_2>\mathrm{F}>\mathrm{COOH}>\mathrm{Cl}>\mathrm{Br}>\mathrm{I}>\mathrm{OH}\)
In contrast, if the substituent bonded to the carbon atom is electron donating (being less electronegative titan carbon) it acquires an 8+ charge and produces a +1 effect.
Some electron-donating groups in decreasing order of +1 effect are:
⇒ \(\mathrm{O}^{-}>\mathrm{CO}_2^{-}>\left(\mathrm{CH}_3\right)_3 \mathrm{C}>\left(\mathrm{CH}_3\right)_2 \mathrm{CH}>\mathrm{CH}_3 \mathrm{CH}_2>\mathrm{CH}_3\)
The inductive effect is transmitted along the chain of carbon atoms but the charge developed on all the carbon atoms in the chain decreases as the distance from the source increases.
In propyl chloride, the partial positive charge developed on C1 induces some positive charge on C2 and C3 gains still less positive charge.
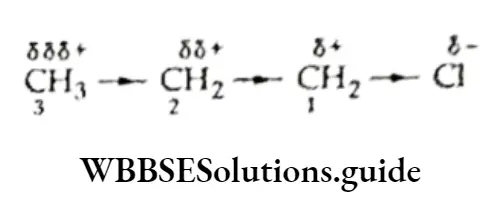
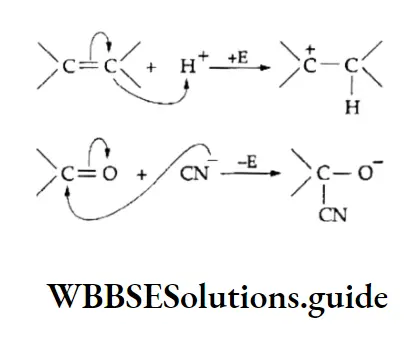
The inductive effect is represented by an arrow pointing towards the electron-withdrawing atom or group, Electromeric effect When a reagent attacks a compound containing a multiple bond (double or a triple bond), n electrons are transferred completely from one atom to the other in the bond.
The atom that gains the electron pair becomes negatively charged and becomes positively charged. This is the electromagnetic effect.
The electromagnetic effect is temporary. The influence of an attacking reagent causes a polarisation in the substrate molecule.
As soon as the reagent is removed, the polarised molecule reverts to its original state.

The electromeric effect is represented by the symbol E and is said to be +E when the displacement of electrons is towards the atom to which the attacking reagent gets attached.
The effect is -E when the electrons are transferred to that atom to which the attacking reagent does not get attached. The curved arrow shows the displacement of the shared electron pair.
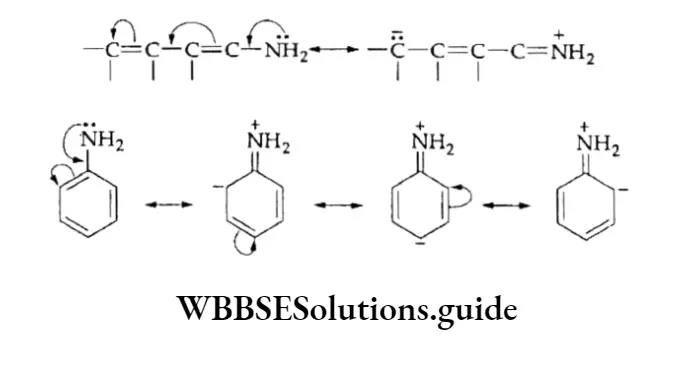
Mesomeric or resonance effect Like the inductive effect, the mesomeric effect also causes permanent polarisation in a molecule.
However, unlike the inductive effect which operates in molecules having single bonds (sigma bonds), the mesomeric or conjugative effect operates only in those molecules which have unsaturated conjugated systems.
A conjugated system in a molecule can be described as a double or triple bond separated by one single bond. In such molecules the delocalisation of some n.
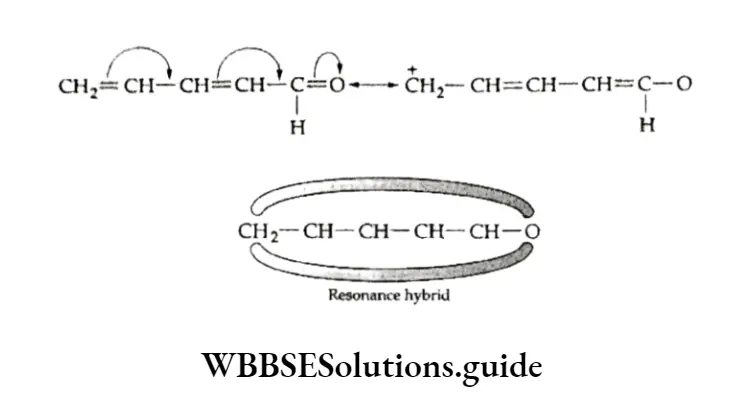
orbital electrons occur between carbon atoms linked by a single bond. Consider the case of a carbonyl group. Its structural formulae I and II do not satisfactorily explain all its properties.
This led to the idea that it exists in a state which is some combination of two or more electronic structures. This is the resonance hybrid (HI) in which electrons are drawn partially towards oxygen.
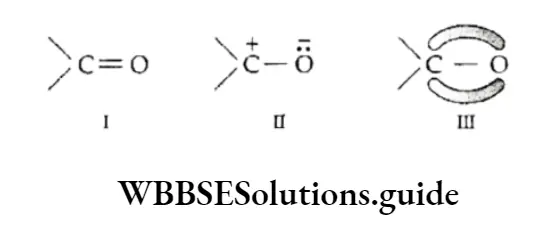
The mesomeric effect is more pronounced if the carboxyl group is conjugated with an unsaturated system such as CH3=CH—CH=CH—CH=0. The TI electrons of the C—O bond get displaced towards oxygen (which is more electronegative) giving rise to the resonance structures.
The resonance structure which has more dispersal of charge (negative charge on the more electronegative atom and positive charge on the more electropositive atom) is more stable.
As explained above, polarization in a molecule is transmitted via the n electrons. The mesomeric effect (denoted by M) can be +M or -M. A group of atoms causes a -M effect when the direction of displacement of electrons is towards it. Examples of groups that cause a -M effect are
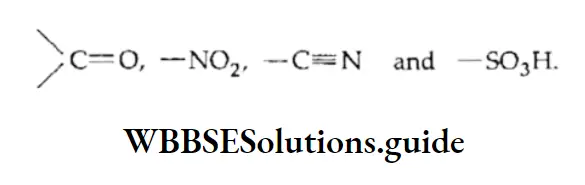
The -M effect of the NO2 group is shown as follows.
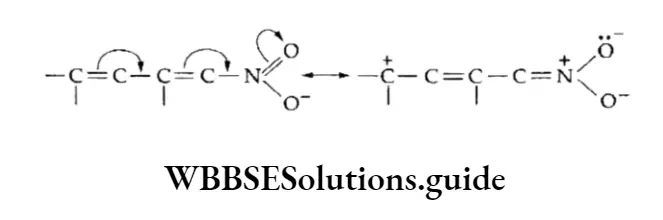
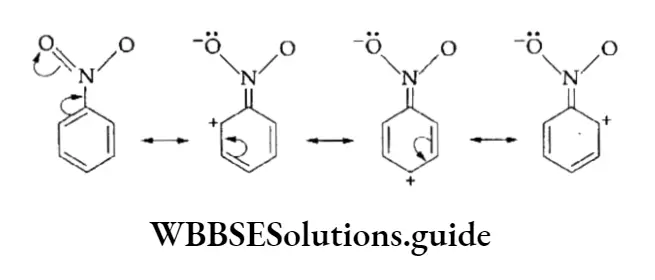
This effect can also be seen in a cyclic system with alternate single and double bonds, for example, nitrobenzene.
A group of atoms has a +M effect if the electron pair is displaced away from it. Some examples of electron-repelling groups are as follows.
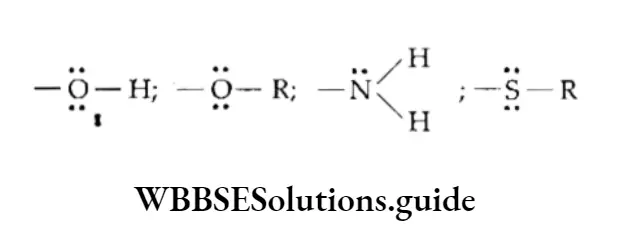
These groups have one or more lone pairs of electrons which they furnish for conjugation with the attached unsaturated system. For example, the NH2 group, which has a lone pair of electrons, shows a +M effect.
This kind of electron transfer is called mesomeric or resonance effect. Recall that when the structure of a molecule can be represented by two or more conventional formulae, the phenomenon is known as resonance.
Hyperconjugation When an H—C bond is attached to an unsaturated n system (double or triple bond) which has an unshared p orbital, there is a conjugation.
In other words, the electrons of the H —C bond become less localised by entering into partial conjugation with the attached unsaturated system.
The interaction of and n electrons is made possible by the partial overlap of orbitals: sp3—s (C—H bond) with the empty p orbital of an adjacent positively charged carbon atom.
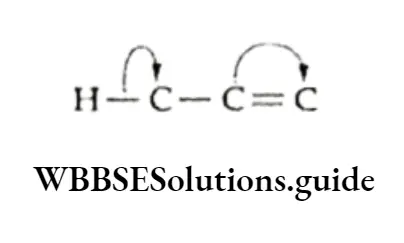
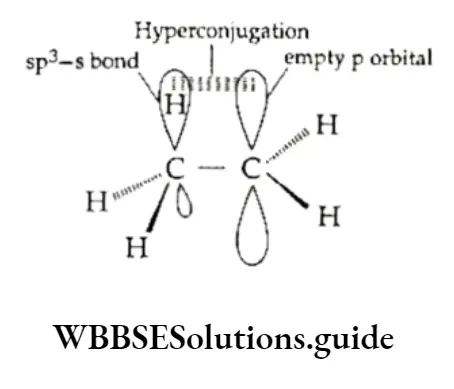
Hyperconjugation stabilises a molecule, for example, propene is more stable than expected.
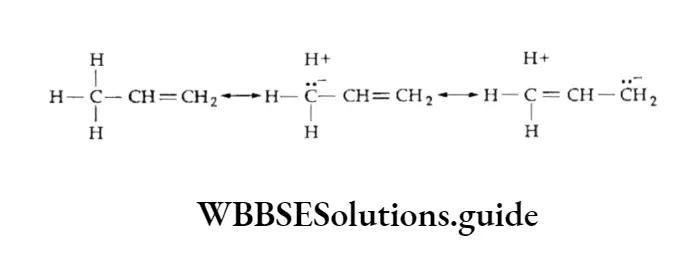
Here the electrons of the C —H bond become partially delocalised through conjugation so that the hydrogen atoms are not free —only the ionic character of the C—H bond is increased. This is one way of looking at the hyperconjugative effect. Hyperconjugation may also be termed “no bond resonance”. It is a permanent effect.
Bond Fission
You already know from your previous classes that when a chemical reaction occurs the bonds in the reactant molecules are broken to form new ones in the products.
The whole mechanism of the reaction depends on the way these bonds are broken. The fission of a covalent bond can take place in two ways—homolytic and heterolytic.
The way in which the bond is broken depends more on the nature of the atoms constituting it and to some extent on the conditions prevailing during the reaction.
Bond fission leads to the formation of reaction intermediates, which are of transitory existence. Most organic reactions take place via the formation of reaction intermediates.
Being very reactive, reaction intermediates may or may not be isolated under normal conditions.
However, their structures have been established by indirect means either chemically or spectroscopically.
The most common reaction intermediates that we come across usually at the present level are carbocations, carbanions and free radicals.
Homolytic bond fission
When, during the fission of a bond, the separating atoms take away one electron each of the shared pair, the fission is symmetrical and called homolytic fission or homolysis.
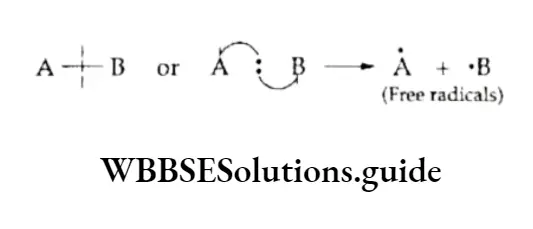
Curved arrow notations are used to represent the shift of electrons. A single electron shift is represented by a half arrowhead (fish arrow).
The two fragments, which may be atoms or groups of atoms, obtained as a result of homolytic bond fission carry an unpaired electron each and are called free radicals.
These are highly reactive reaction intermediates and tend to pair up or react with other radicals or molecules to restore stability by being a part of the bonding pair.
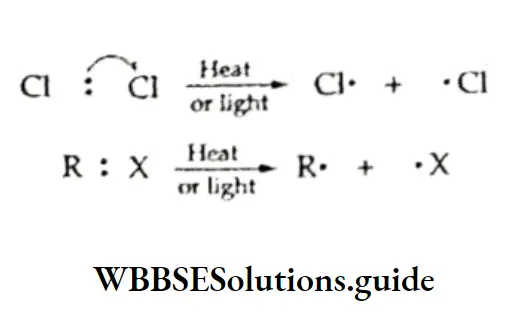
Free radicals can be produced by photolysis or pyrolysis in which a bond is broken and ions are not formed.
For example, the cleavage of a chlorine or an alkyl halide molecule takes place in the presence of ultraviolet light or heat giving rise to two chlorine and one alkyl and one halide free radical respectively.
Alkyl free radicals can be classified as primary, secondary or tertiary, depending upon which hydrogen (1°, 2°, or 3°) is abstracted from the alkyl. Since the ease of abstraction of hydrogen atoms follows the sequence 3° > 2° > 1° > CH 4, the ease of formation (and stability) of free radicals follows the sequence:
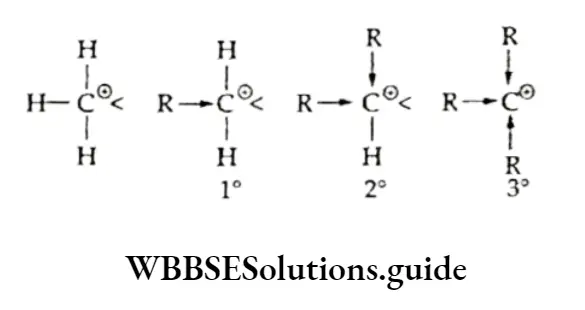
Titus, the stability of alkyl free radicals increases from primary to tertiary. Organic reactions which have free radicals as intermediates are called free-radical or nonpolar reactions.
Heterolytic bond fission
In heterolytic cleavage, when the bond between two atoms breaks, both the electrons of the shared pair are taken over by one of the atoms.
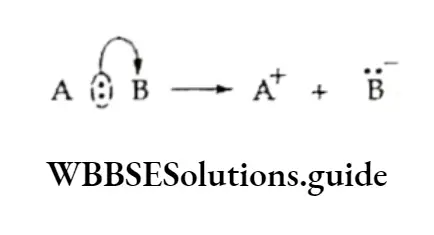
Heterolytic cleavage is represented by a curved arrow that starts from the bonded pair of electrons and points at the atom which retains the shared pair of electrons.
Thus a heterolytic bond fission gives rise to one positive species, which lacks a pair of electrons in its valence shell and one negative species with a valence octet.
The more electronegative atom retains the shared pair of electrons. Heterolytic bond fission in organic molecules leads to the formation of either carbocations or carbanions.
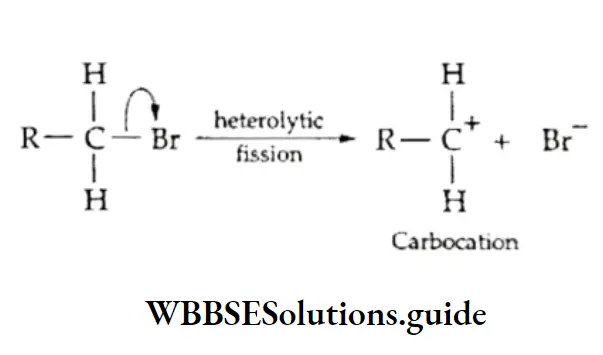
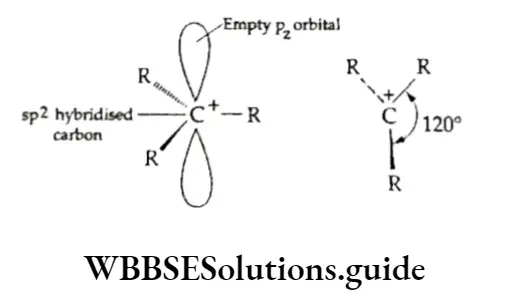
In a carbocation, the carbon atom carries a positive charge, i.e., lacks a pair of electrons in its valence shell. In contrast, the carbanion, as the name suggests, is a negative group in which the carbon atom carries a negative charge, i.e., has an unshared pair of electrons.
The positively charged carbon atom with a sextet of electrons is sp2 hybridised and it uses all its three hybridised orbitals to form bonds with other atoms. The remaining pz orbital is empty and is perpendicular to the plane of the molecule.
The shape of a carbocation may be described as planar (trigonal coplanar) with a bond angle of 120°
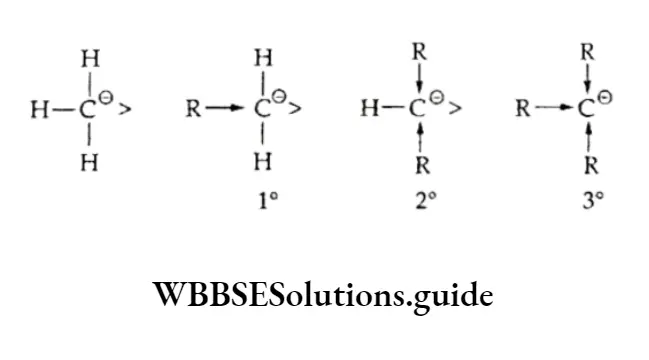
Carbocations are also classified as primary (1°), secondary (2°) or tertiary (3°) depending on whether one, two or three carbon atoms respectively are attached to the carbon bearing the positive charge.
Since a carbocation has a positively charged (electron deficient) carbon, its relative stability is determined chiefly by how well it accommodates that charge.
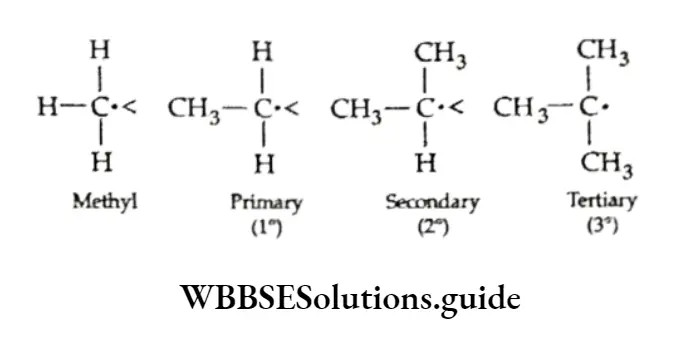
Dispersal of the charge leads to the stability of the charged system. (This applies equally well to both positively and negatively charged systems.)
The greater the number of alkyl groups attached to a positively charged carbon atom, the greater the charge dispersion due to hyperconjugation lending to a greater stability of the carbocation.
In the hyperconjugative effect, electrons are provided by o bonds to the empty p orbital of the electron-deficient carbon. Thus, the order of stability of carbocations is
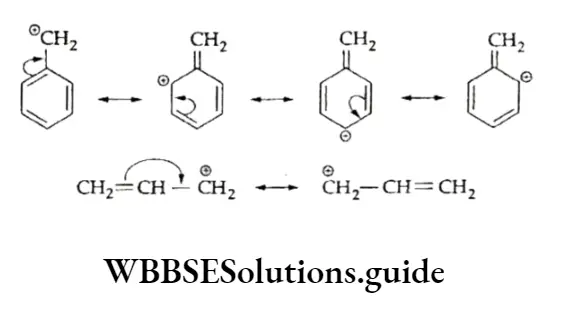
The arrows pointing towards the electron-deficient carbon indicate electron release from the alkyl groups. Benzyl and allyl carbocations, though primary in nature, are stable due to the resonance effect.
The greater the number of canonical structures for a carbocation, the more stable it is. In view of this, the order of stability of the carbocations is
⇒ \(\mathrm{C}_6 \mathrm{H}_5 \stackrel{\ominus}{\mathrm{C}} \mathrm{H}_2>\mathrm{CH}_2=\mathrm{CH}-\stackrel{\ominus}{\mathrm{C}} \mathrm{H}_2>\mathrm{CH}_3 \mathrm{CH}_2 \stackrel{\stackrel{\circ}{\mathrm{C}} \mathrm{H}_2}{ }\)
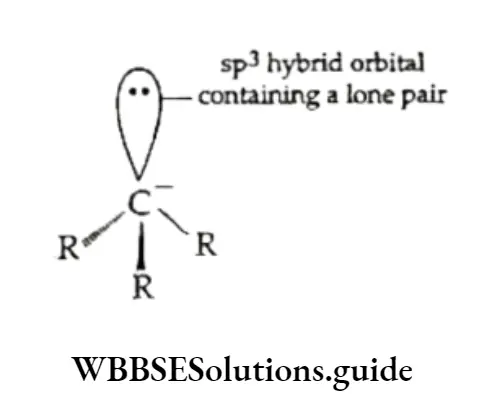
A carbanion is a species containing a carbon atom with one pair of unshared electrons and thus carrying a negative charge.
The carbanion is pyramidal and the central carbon atom is sp3 hybridised with the unshared or lone pair of electrons occupying one sp3 hybrid orbital.
The order of stability of carbanions is just the reverse of that in carbocations. This can be explained on the basis of the +1 (inductive) effect of the alkyl group.
Being electron-releasing, the alkyl group tends to increase the electron density on the negatively charged carbon atom, lowering its stability. Thus, the order of stability of carbanions is
Types Of Reagents
As already stated, generally an organic reaction proceeds when a reagent attacks a substrate molecule.
Under suitable reaction conditions and the influence of an attacking reagent, the substrate molecule undergoes bond fission to form reaction intermediates, which combine with the reagent to give the final product.
Attacking reagents may be of two types—electrophilic and nucleophilic, depending on the electron density at the attacking centre.
Electrophilic reagents
These are electron-loving species. Being electron-deficient, they attack the electron-rich centre in the substrate molecule—that specific atom in the substrate molecule which is electron-rich. Electrophiles (E+) include positively charged species and neutral molecules.
For example, H+, H30+ and carbocations (CH3 ,RH2C+). Neutral molecules like A1C13, BF3 and SO3 act as electrophiles as they are electron-deficient.
Molecules having polarisable functional groups like the carbonyl group (ÿC=0) and alkyl halides (halogen atom is polarised) also act as electrophiles.
In alkyl halides, due to the polarity of the C—X bond, a partial positive charge develops on the carbon atom, which becomes an electrophilic centre
Nucleophile reagents
These reagents are nucleus-loving and attack electron-deficient centres in the substrate molecule. Nucleophiles (Nu:) may be negatively charged ions or neutral molecules with a lone pair of electrons. For example, Cl~,OH_,CN+ and carbanions (R3C:).
Neutral molecules containing a lone pair of electrons such as H2O, R3N, NH3, ROH and ROR act as nucleophiles.
As stated earlier, the mechanism of organic reactions, apart from the reactivity of the substrate, the nature of bond fission and reagents, also depends on the type of reaction that occurs.
You will come across different types of reactions while studying the preparation and properties of hydrocarbons.
Purification Of Organic Compounds
To study the structure and properties of an organic compound, it is essential to obtain the compound in its purest form.
An organic compound isolated from various sources contains many types of impurities.
Many methods are used to purify organic compounds. The choice of the purification method employed depends on the type of impurities present and the nature of the organic compound.
The common methods of purification are as follows.
- Crystallisation
- Distillation
- Differential extraction
- Sublimation
- Chromatography
Crystallisation
Crystallisation is the process of the formation of a crystalline solid from a solution, generally by the evaporation of the solvent.
The process is most commonly used for the purification of solid organic compounds. The method is based on the difference in the solubilities of the compound and the impurities in a suitable solvent.
The choice of the solvent is very important. A solvent which dissolves less of the organic compound at room temperature and an appreciable quantity at a higher temperature is suitable for preparing the solution.
Also, the solvent should not dissolve the impurities and should not react with the compound. A hot saturated solution is filtered and then allowed to cool down slowly to room temperature when the compound separates in the form of crystals.
In case any coloured impurities are present in the organic compound, crystallisation is carried out by heating the hot saturated solution with a small amount of activated charcoal, which adsorbs the impurities.
“Organic Chemistry, Some Basic Principles and Techniques, important questions”
Repeated crystallisation of a compound from a solvent to obtain the increasingly pure form is called recrystallisation.
It is generally done for the purification of compounds containing impurities of comparable solubilities. A mixture of two or more compounds can be separated by fractional crystallisation.
The method is based on the different solubilities of compounds in the same solvent. The less soluble compound separates out first while the more soluble one remains in the solution.
A mixture of benzoic acid and cane sugar can be separated by this method. Benzoic acid is less soluble in water than cane sugar.
Distillation
The process is used to separate liquids with appreciable differences in their boiling points. Also, a volatile liquid can be separated from nonvolatile impurities.
The process involves the conversion of the liquid into its vapours by heating followed by condensation of the vapours to obtain the pure liquid.
Distillation is of the following types:
- Simple distillation
- Fractional distillation
- Distillation under reduced pressure, or vacuum distillation
- Steam distillation
The choice of the distillation process depends on the nature of the liquids to be separated and the kind of impurities present in them.
Simple distillation
This method is used for the purification of liquids which boil without decomposition and contain nonvolatile impurities. The apparatus consists of a distillation flask with a side tube, connected to a Liebig condenser, through which water is passed.
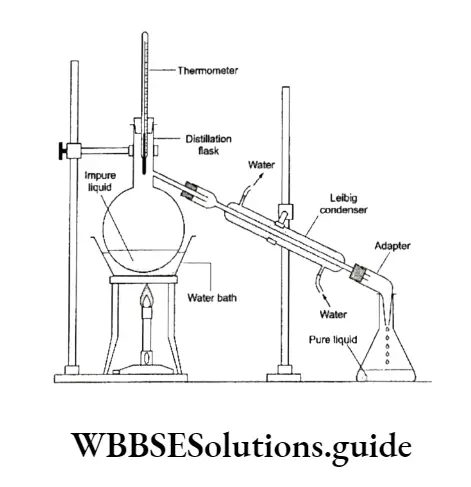
The liquid to be purified is taken in the distillation flask. A few pieces of glass beads or unglazed porcelain are put in the flask.
This helps to avoid bumping (violent boiling of a liquid caused by super-heating) during distillation. For a liquid with a low boiling point, say 353-363 K, the flask is heated on a water bath and for a liquid with a high boiling point, say more than 373 K, an oil bath is used.
In place of the oil bath, the distillation flask can also be heated with a small flame over a wire gauze.
On heating, the liquid changes to vapours, which are passed through a condenser where they condense into liquid.
The pure distilled liquid is collected and the impurities are left behind in the distillation flask. The constant temperature at which most of the liquid distils is known as the boiling point of that liquid.
Fractional Distillation
Simple distillation cannot be used for the separation and purification of liquids with comparable boiling points. This is because both the liquids will be distilled simultaneously.
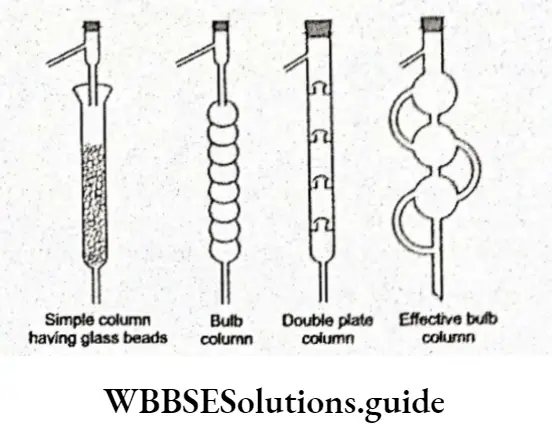
The separation of such liquid mixtures can be achieved by employing fractional distillation. This involves the use of a suitable fractionating column. Different types of fractionating columns can be used.
Basically, a fractionating column is a long tube filled with glass beads or a tube in which the walls have many inward-pointing indentations.
The fractionating column obstructs the passage of the vapours moving upwards and the liquid moving downwards. In fact, the indentations increase the cooling surface.
The fractionating column is fitted onto the mouth of a round bottom flask, which contains the liquid mixture.
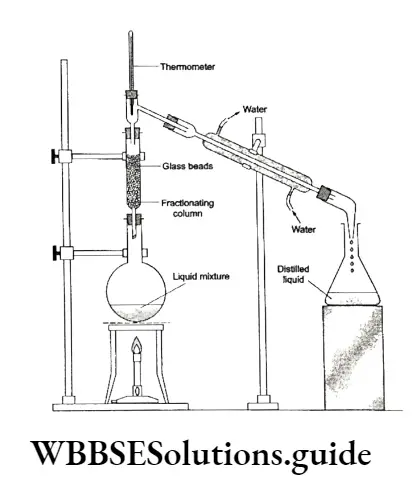
When the flask is heated, the vapours of the more volatile liquid rise up in the fractionating column and the less volatile fraction condenses back into the flask (higher up the column, the temperature being lower).
As the vapour mixture rises up, it becomes rich in the low-boiling (volatile) component.
Thus, by the time the low-boiling vapours reach the top of the column, they become concentrated and pure. These vapours then pass through the condenser and pure liquid is collected.
After many successive distillations, the remaining liquid in the flask gets enriched in the high-boiling component.
Each successive condensation and vaporisation unit in the fractionating column is called a theoretical plate.
The temperature at the top of the fractionating column indicates the fraction being distilled.
Industrial applications of fractionating columns are in petroleum refineries, petrochemical plants and natural gas processing plants. This method is also used to separate the components of air.
Distillation under reduced pressure (vacuum distillation)
This method is used for the separation and purification of liquids which have high boiling points and those which decompose on heating. The distillation is carried out under reduced pressure.
The depression in the boiling point of the substance distilled means that the temperature is lower, which prevents the substance from decomposing. Low pressure can be achieved by using a vacuum pump.
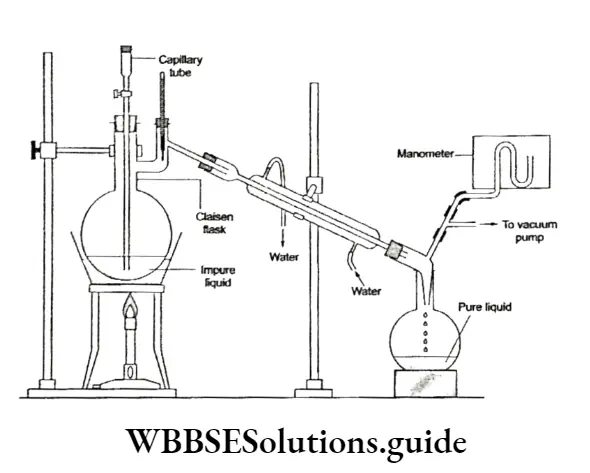
The apparatus used in this method is more or less of the same type as that used in simple distillation except that instead of a round bottom flask, a two-necked flask, called the Claisenflask, is used.
In one of the necks, a capillary tube is fitted, which allows air bubbles to pass through (to prevent bumping) and in the second neck, a thermometer is fitted.
The pressure under which the liquid distils is measured with the help of a manometer.
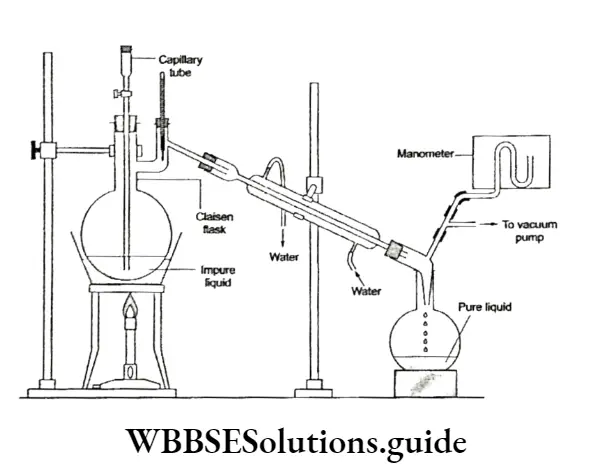
A high-boiling liquid like glycerol (b.p. 563 K) can be easily separated from spent lye in the soap manufacturing process by this method.
Spent lye is a mixture of brine and glycerine, which is separated from crude soap during soap manufacturing. Another application of vacuum distillation is in the concentration of sugar cane juice.
Steam distillation
This method is used for the purification of those compounds which are volatile in steam, immiscible with water, and which contain nonvolatile impurities and possess a high vapour pressure.
In this process, the liquid to be purified is taken in a distillation flask and steam is continuously bubbled through it. The steam is generated using a steam generator.
The distillation flask is also heated so as to maintain the temperature at about 373 K. Initially, the steam heats the liquid but itself gets condensed.
But after some time, the mixture begins to boil. You already know that a liquid boils when its vapour pressure is equal to the atmospheric pressure.
In this case, water and the organic substance vaporise together and the total vapour pressure of the two becomes equal to the atmospheric pressure.
This implies that in steam distillation, the organic substance (being steam volatile) vapourises and gets distilled at a lower temperature than the boiling point, thus avoiding decomposition.
Thus, steam distillation serves the same purpose as distillation under reduced pressure.
The organic liquid is separated from water using a separating funnel. A mixture of water and aniline can be separated by this method. The process is also employed in the manufacture of essential oils.
Differential Extraction
This method is used to isolate or recover organic compounds (liquids or solids) present in an aqueous solution.
In this process, an aqueous solution of the organic compound is taken in a separating funnel, and mixed with a suitable organic solvent. The mixture is shaken.
The solubility of the organic compound should be higher in the organic solvent than in water. Also, the organic solvent should be immiscible with water.
Ether, benzene, chloroform, ethyl acetate, etc., are some of the common organic solvents used for this purpose.
When the contents are shaken thoroughly in the stoppered separating funnel, the organic phase dissolves the compound present in the aqueous phase.
The separating funnel is then allowed to stand for some time when the aqueous and the organic phase (solvent) separate as distinct layers. The two layers are extracted one after the other by opening the stop code, thus the phenomenon is known as differential extraction.
The process is repeated 2-3 times till all the organic compound originally dissolved in the aqueous layer is extracted with the organic solvent.
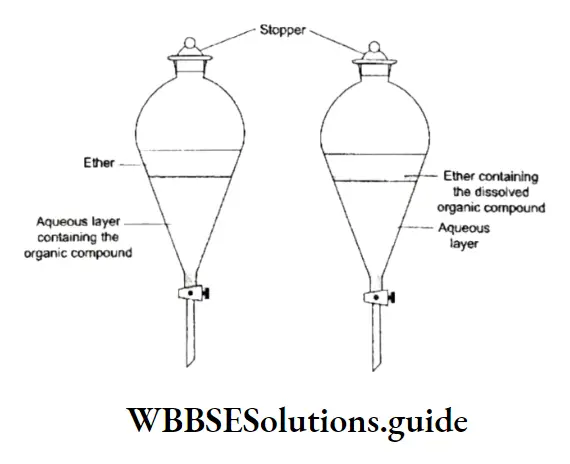
The organic compound is separated from the solvent by distillation. If the solubility of the organic compound is less in the solvent, then a large quantity of the solvent is used to extract a small quantity of the compound.
In that case, the same solvent is employed repeatedly. This technique is known as continuous extraction.
The method of differential extraction is useful for the isolation of nonvolatile compounds.
For example, benzoic add, which is soluble in water, can be extracted by the extraction of the aqueous solution using ether as the solvent.
Sublimation
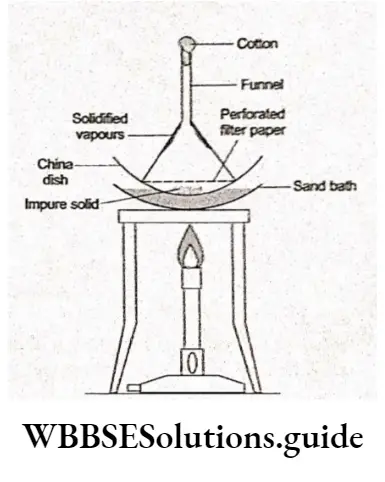
On heating, some organic compounds change from the solid to the vapour state directly without passing through the intervening liquid state.
This process is called sublimation. Only those substances sublime whose vapour pressures equal the atmospheric pressure much before their melting points.
This process is useful for the purification of such solids which sublime on heating and contain nonvolatile impurities.
In this process, the impure solid is heated in a china dish which is covered with an inverted funnel.
A perforated filter paper is folded in the shape of the funnel and adjusted on the inner surface of the funnel. The stem of the funnel is plugged with cotton.
On heating, the substance volatilises and the vapours rise, solidify and get deposited on the inner, less hot surface of the inverted funnel. The non-volatile impurities are left in the china dish.
The perforations in the filter paper allow only the vapours to pass through it. The compounds which can be purified by sublimation are camphor, naphthalene and anthracene.
Chromatography
Chromatography is a method of separation in which the compounds to be separated are in two phases, one of which is stationary (stationary phase) while the other (mobile phase) moves in a definite direction.
The method was first used to separate coloured pigments found in plants, hence the name (Greek, Chroma-colour).
Generally speaking, the different chromatographic techniques work on either of the two principles— the difference between adsorption affinities and solubilities of the various components of the mixture.
Based on this, we shall study the two categories of chromatography—adsorption chromatography and partition chromatography.
Adsorption chromatography
Chromatography in which separation is based mainly on the differences between the adsorption affinities of the components in a sample for the surface of an active solid is called adsorption chromatography.
A porous solid with adsorptive properties which help in chromatographic separations is known as an active solid.
Here the stationary phase is the adsorbent (active solid, e.g., alumina). Thus the principle involved is differential adsorption.
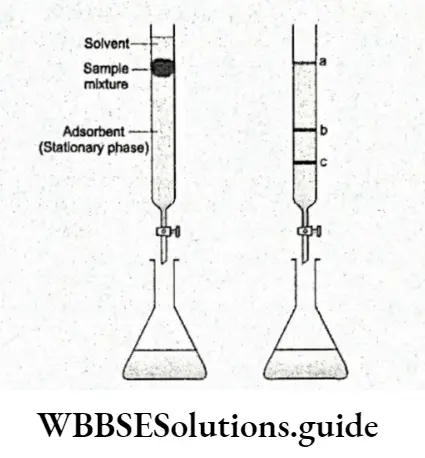
The two main types of chromatography based on the principle of differential adsorption are column chromatography and thin-layer chromatography. Column chromatography The stationary bed, in this technique, is within a tube.
The adsorbent or solid stationary phase may fill the inside of the tube or be concentrated along the inside tube wall leaving an unrestricted path for the mobile phase in the middle part of the tube. The most commonly used adsorbents are alumina and silica gel.
Once the adsorbent is packed in the glass tube, the sample is poured into the column and continuously washed with a solvent a process known as elution. The solvent is called an elu
Different components of the sample are adsorbed to different extents and move down the column at different rates. Thus the most readily adsorbed components are at the top of the column.
The solution is the mobile phase in this case. The usual method is to collect the solution as it passes out from the column in fractions.
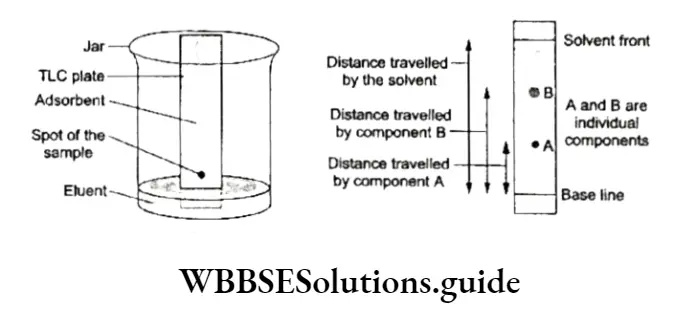
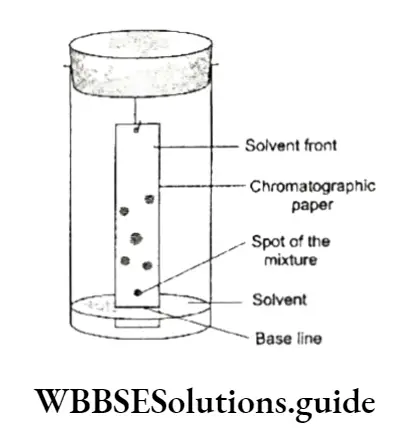
Thin-layer chromatography (TLC) Chromatography is carried out on a layer of adsorbent spread on a glass plate.
The layer is thin (about 0.2 mm) and known as a thin-layer chromatography plate or chrome plate. A small spot of the solution of the mixture is put on the plate at a distance of about 2 cm from the base.
This is done with the help of a capillary tube. The plate is then dried and placed in a glass jar containing the eluent at the bottom. It is ascertained that the spot on the TLC plate does not touch or dip in the eluent. The jar is covered with a glass plate.
The eluent moves up the plate due to capillary action and meets the sample mixture. The components of the mixture get dissolved in the solvent (eluent) and are carried up the plate.
The components of the mixture get separated into a number of spots due to their different adsorption affinities. Each component corresponds to a component of the mixture.
The plate is taken out, and the solvent front is marked and dried. Each component is finally eluted with a suitable solvent to get the pure component.
The relative adsorption of each component of the mixture is denoted by its retention factor (Rÿ).
The Rj values of a spot can be determined by dividing the distance travelled by that spot (component) by the total distance travelled by the solvent (solvent front). The distances are measured from the baseline.
The spots of coloured compounds are visible on the TLC plate but analysing colourless compounds becomes difficult. There are several methods to make the colourless spots visible.
If a small amount of fluorescent dye is added to the adsorbent, ultraviolet radiation absorbing, colourless spots can be seen. Iodine vapours are also used as the colour reagent.
Specific colour reagents are also sprayed on the TLC plate. For instance, ninhydrin gives a yellow colour when it reacts with the amino add proline and a blue colour with other amino acids.
Partition Chromatography
In this type of chromatography, the separation of the components of the mixture is based mainly on the differences between their solubilities in the stationary and mobile phases.
Paper chromatography is a type of partition chromatography. In this case, a strip of chromatographic paper acts as the adsorbent. The molecules of water trapped in the chromatographic paper serve as the stationary phase. The mobile phase is the suitable solvent or a mixture of solvents.
A spot of the mixture to be separated is placed near one edge of the paper and the sheet is suspended vertically in a solvent, which rises through the paper by capillary action, carrying the components with it. The components of the mixture move at different rates and separate.
The adsorption of components to different extents on the paper and the partition between the solvent and the moisture in the paper are the two factors responsible for the separation of the components of the mixture.
The paper is removed, the solvent front is marked, and the paper is dried. The dried chromatographic paper with a line of spots of different components of the mixture is called a chromatogram.
The components can be identified by the distance they move in a given time. Colourless substances are detected by using ultraviolet radiations or by spraying with a substance which reacts to give a coloured spot (substance).
Rf can be calculated for each component. If for a given system at a known temperature is a characteristic of the component and can be used to identify compounds.
Qualitative Analysis
Once the compound has been purified, its structure can be determined. To do so, it is necessary to identify the elements constituting the compound. This is known as qualitative analysis.
You already know that organic compounds invariably contain carbon and hydrogen. Besides, they may also contain nitrogen, sulphur, a halogen and phosphorus. The presence or the absence of these elements is ascertained as follows.
Detection Of Carbon And Hydrogen
The presence of carbon and hydrogen is detected by strongly heating the compound with cupric oxide.
The carbon in the compound is oxidised to carbon dioxide (tested by passing the gas through limewater) and hydrogen is converted into water (tested by using anhydrous copper sulphate, which turns blue).
⇒ \(\begin{aligned}
& \mathrm{C}+2 \mathrm{CuO} \longrightarrow \mathrm{CO}_2+2 \mathrm{Cu} \\
& 2 \mathrm{H}+\mathrm{CuO} \longrightarrow \mathrm{H}_2 \mathrm{O}+\mathrm{Cu}
\end{aligned}\)
⇒ \(\mathrm{CO}_2+\mathrm{Ca}(\mathrm{OH})_2 \longrightarrow \mathrm{CaCO}_3 \downarrow+\mathrm{H}_2 \mathrm{O}\)
⇒ \(
\begin{aligned}
& \mathrm{C}+2 \mathrm{CuO} \longrightarrow \mathrm{CO}_2+2 \mathrm{Cu} \\
& 2 \mathrm{H}+\mathrm{CuO} \longrightarrow \mathrm{H}_2 \mathrm{O}+\mathrm{Cu} \\
& \mathrm{CO}_2+\mathrm{Ca}(\mathrm{OH})_2 \longrightarrow \mathrm{CaCO}_3 \downarrow+\mathrm{H}_2 \mathrm{O} \\
& \mathrm{H}_2 \mathrm{O}+\underset{\text { (White) }}{\mathrm{CuSO}_4} \longrightarrow \underset{\text { (Blue) }}{\mathrm{CuSO}_2 \cdot 5 \mathrm{H}_2 \mathrm{O}}
\end{aligned}
\)
Detection Of Other Elements
Apart from carbon and hydrogen, the other elements generally present are detected by Lassaigne’s test.
The compound to be analysed is fused with sodium metal. The elements covalently bonded to the carbon or the hydrogen atoms in the molecule of the compound get converted into water-soluble, ionic salts. The conversion to ionic forms makes the detection of elements easier.
⇒ \(Organic compound +\mathrm{Na} \longrightarrow \mathrm{NaCN}+\mathrm{Na}_2 \mathrm{~S}+\mathrm{NaX}
(C, H, O, N, S, X)\)
In case both nitrogen and sulphur are present in the same molecule, sodium thiocyanate (NaSCN) is obtained on fusion with sodium. The ionic salts arc extracted from the fused mass by boiling it with distilled water.
The solution obtained is the sodium extract or Lassaignc’s extract, which is then used to detect the elements.
Test for nitrogen
The sodium extract is heated to boiling with a few crystals of ferrous sulphate and then acidified with dilute sulphuric acid, and 2-3 drops of ferric chloride solution are added.
If nitrogen is present, a Prussian blue precipitate of ferric ferrocyanide is obtained. The blue colour is due to the cyanide ions.
Hydrazine (H2N—NH2) cannot be detected by Lassaigne’s test due to the presence of the N—N bond.
⇒ \(2 \mathrm{NaCN}+\mathrm{FeSO}_4 \longrightarrow \mathrm{Fe}(\mathrm{CN})_2+\mathrm{Na}_2 \mathrm{SO}_4\)
⇒ \(
\begin{gathered}
\mathrm{Fe}(\mathrm{CN})_2+4 \mathrm{NaCN} \longrightarrow \underset{\text { Sodium hexacyanoferrate(II) }}{\mathrm{Na}_4\left[\mathrm{Fe}(\mathrm{CN})_6\right]} \\
3 \mathrm{Na}_4\left[\mathrm{Fe}(\mathrm{CN})_6\right]+4 \mathrm{FeCl}_3 \longrightarrow \underset{\text { Ferriferrocyanide }}{\mathrm{Fe}_4\left[\mathrm{Fe}(\mathrm{CN})_6\right]_3+12 \mathrm{NaCl}}
\end{gathered}\)
The presence of nitrogen in a compound can also be detected by the soda lime test. The compound is heated with soda lime —the evolution of ammonia confirms the presence of nitrogen.
⇒ \(\underset{\text { Actamide }}{\mathrm{CH}_3 \mathrm{CONH}_2+\mathrm{NaOH}} \underset{\text { theat }}{\stackrel{\mathrm{CnO}}{\longrightarrow}} \mathrm{CH}_3 \mathrm{COONa}+\mathrm{NH}_3\)
The evolved ammonia can be tested by bringing a glass rod dipped in concentrated HC1 to the mouth of the test tube. White fumes of NH4C1 are seen.
Test for sulphur
Acetic acid followed by a lead acetate solution is added to the sodium extract. A black precipitate indicates the presence of sulphur.
⇒ \(\left(\mathrm{CH}_3 \mathrm{COO}\right)_2 \mathrm{~Pb}+\mathrm{Na}_2 \mathrm{~S} \stackrel{\mathrm{H}^{+}}{\longrightarrow} \underset{\text { (black) }}{\mathrm{PbS} \downarrow}+2 \mathrm{CH}_3 \mathrm{COONa}\)
Alternatively, when sodium nitroprusside solution is added to the sodium extract, a violet colour indicates the presence of sulphur.
⇒ \(\underset{\text { Sodium nitroprusside }}{\mathrm{Na}_2 \mathrm{~S}}+\underset{\mathrm{Na}_2\left[\mathrm{Fe}(\mathrm{CN})_5 \mathrm{NO}\right]}{\longrightarrow} \underset{\text { violet }}{\mathrm{Na}_4\left[\mathrm{Fe}(\mathrm{CN})_5 \mathrm{NOS}\right]}\)
In case sulphur is present along with nitrogen, then the sodium extract will contain sodium thiocyanate, which instead of Prussian blue gives a blood red colour with ferric chloride due to the presence of the thiocyanate.
⇒ \(3 \mathrm{NaSCN}+\mathrm{FeCl}_3 \longrightarrow \underset{\substack{\text { Ferrithiocyanate } \\ \text { (blood red) }}}{\mathrm{Fe}(\mathrm{SCN})_3}+3 \mathrm{NaCl}\)
However, with excess sodium, during sodium fusion, the thiocyanate decomposes to yield cyanide and sulphide, which respond positively to their usual tests.
⇒ \(\mathrm{NaSCN}+2 \mathrm{Na} \longrightarrow \mathrm{NaCN}+\mathrm{Na}_2 \mathrm{~S}\)
Test for halogens
The sodium extract is boiled with dilute nitric acid to convert sodium cyanide or sodium sulphide (if present) to hydrogen cyanide and hydrogen sulphide respectively.
The cyanide or sulphide ions otherwise interfere with further tests. The cooled solution is treated with silver nitrate.
A white precipitate soluble in ammonium hydroxide confirms the presence of chlorine.
A yellowish precipitate sparingly soluble in ammonium hydroxide indicates bromine while a yellow precipitate insoluble in ammonium hydroxide confirms the presence of iodine.
The presence of bromine and iodine can also be detected by using carbon disulphide or carbon tetrachloride.
The sodium extract is boiled with dilute H2S04, cooled, and chlorine water followed by CS2 or CC14 is added.
The solution is shaken and allowed to stand. The layer of CS2 or CC14 (organic layer) turns yellow or orange in the presence of bromine and violet in case iodine is present.
Test for phosphorus
To detect phosphorus, the organic compound is fused with sodium peroxide, which is an oxidising agent.
The phosphorus present is oxidised to phosphate (Na3P04). The aqueous extract containing the phosphate is boiled with concentrated HN03 and a few drops of ammonium molybdate solution are added to it.
A yellow solution or precipitate shows the presence of phosphorus. This yellow precipitate is due to the formation of ammonium phosphomolybdate.
⇒ \(\mathrm{Na}_3 \mathrm{PO}_4+3 \mathrm{HNO}_3 \longrightarrow \mathrm{H}_3 \mathrm{PO}_4+3 \mathrm{NaNO}_3\)
⇒ \(\mathrm{H}_3 \mathrm{PO}_4+12\left(\mathrm{NH}_4\right)_2 \mathrm{MoO}_4+21 \mathrm{HNO}_3 \longrightarrow\left(\mathrm{NH}_4\right)_3 \mathrm{PO}_4 \cdot 12 \mathrm{MoO}_3+21 \mathrm{NH}_4 \mathrm{NO}_3+12 \mathrm{H}_2 \mathrm{O}\)
There is no direct test for the detection of oxygen in an organic compound. Its presence can, however, be inferred by testing for the presence of an oxygen-containing functional group like —OH, —CHO, —COOH and NO2.
Quantitative Analysis
Once the various elements present in an organic compound are detected by qualitative analysis, in order to derive its empirical formula, it is necessary to determine quantitatively the percentages of these elements in the compound.
Estimation Of Carbon And Hydrogen
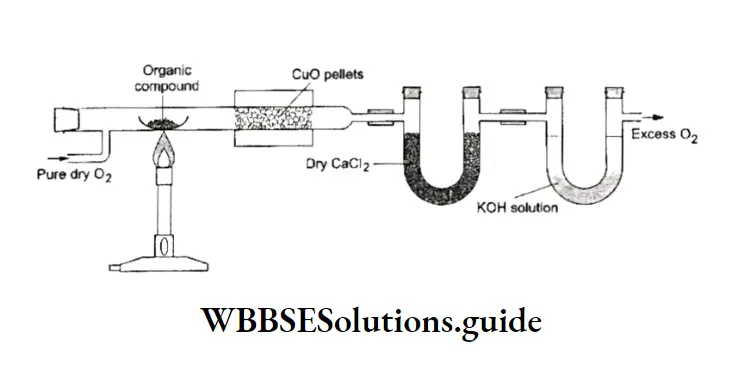
Liebig’s method In this method, a known mass of the organic compound is heated in a current of pure dry oxygen in the presence of cupric oxide till all the carbon and hydrogen are oxidised to carbon dioxide and water respectively.
⇒ \(\text { Organic compound }+\mathrm{O}_2+\underset{\text { excess }}{\mathrm{CuO}} \stackrel{\text { heat }}{\longrightarrow} \mathrm{CO}_2+\mathrm{H}_2 \mathrm{O}+\mathrm{Cu}\)
After combustion, the gases are passed first through a U-tube containing anhydrous calcium chloride, which absorbs water and then through a U-tube containing potassium hydroxide solution, which absorbs carbon dioxide.
Both the U-tubes are weighed before and after the combustion so that the amount of HzO and CO2 evolved from a known amount of the sample can be calculated.
Let the mass of the organic compound be m g.
- Mass of water formed = m1 g.
- Mass of carbon dioxide formed = m2 g.
- Since 44 g of CO2 =12 g of carbon,
- m2 g of CO2 \(\equiv \frac{12}{44} \times m_2\) ni2 g of carbon.
This is the mass of carbon present in m g of the organic compound.
∴ Percentage of C in the compound \(=\frac{12}{44} \times m_2 \frac{100}{m} \text {. }\)
Similarly, the percentage of H in the compound \(=\frac{2}{18} \times m_1 \times \frac{100}{m}.\)
Estimation Of Nitrogen
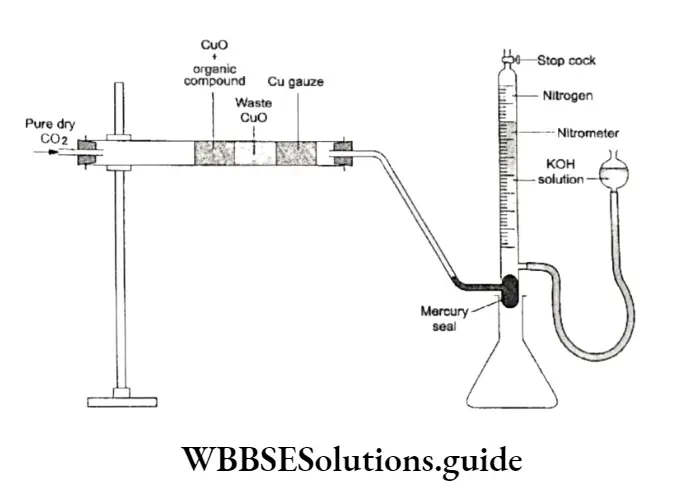
Nitrogen can be estimated either by the Dumas method or by the Kjeldahl method. Dumas method In this method, a weighed sample of the organic compound is heated with cupric oxide in an atmosphere of carbon dioxide.
On heating, the carbon and hydrogen are oxidised to CO2 and H2O respectively, while N2 is set free.
Any oxides of nitrogen produced during combustion are reduced back to nitrogen by passing the sample over a heated copper gauze. The gaseous mixture is collected in a nitrometer, which contains a solution of potassium hydroxide.
The mercury at the bottom of the nitrometer does not allow the potassium hydroxide solution to move from the nitrometer.
All gases except nitrogen are absorbed in the KOH solution. The volume of nitrogen gas collected at the top of the tube is measured.
The percentage of N in the given organic compound is calculated as follows.
- Let the mass of the organic compound = mg.
- The volume of nitrogen collected in the nitrometer = V mL.
- Room temperature = TK
- Pressure of dry nitrogen = (atmospheric pressure- aqueous tension at t° C)
= p bar.
From the relation \(\frac{p_1 V_1}{t_1}=\frac{p_2 V_2}{t_2}\) We can find the volume of N2 gas at stp.
Experimental values
p1 =pbar
V1= VmL
t1 =TK
At stp
p2 = 1bar
V2=?
T2 = 273 K
Thus, \(V_2=\frac{p_1 \times V_1 \times 273}{T \times 1}\)
We know that 22,700 mL of N2 at stp weighs 28 g.
∴ \(V_2 \mathrm{~mL} \text { of } \mathrm{N}_2 \text { at stp will weigh } \frac{28 \times V_2}{22,700} \mathrm{~g} \text {. }\)
Percentage of N = \(\frac{\text { mass of nitrogen }}{\text { mass of organic compound }} \times 100\)
⇒ \(=\frac{28 \times V_2}{22700} \times \frac{100}{m} .\)
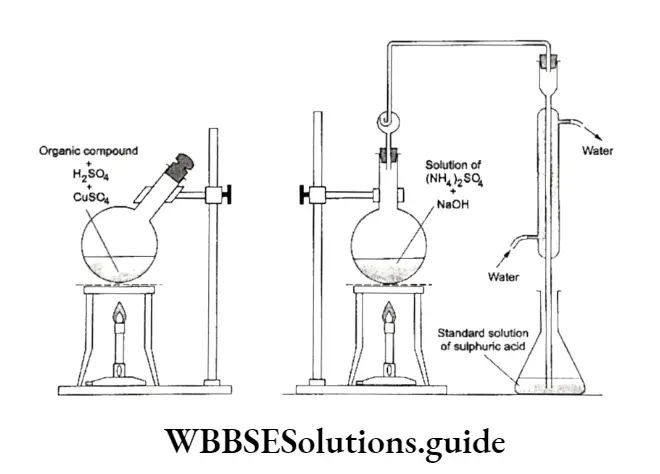
Kjeldahl method In this method a known mass of the organic compound is heated with concentrated sulphuric acid in the presence of copper sulphate, which acts as a catalyst.
The nitrogen of the organic compound gets converted into ammonium sulphate. The solution is then heated with an excess of sodium hydroxide.
⇒ \(\text { Organic compound }+\mathrm{H}_2 \mathrm{SO}_4 \longrightarrow\left(\mathrm{NH}_4\right)_2 \mathrm{SO}_4\)
⇒ \(\left(\mathrm{NH}_4\right)_2 \mathrm{SO}_4+2 \mathrm{NaOH} \longrightarrow \mathrm{Na}_2 \mathrm{SO}_4+2 \mathrm{NH}_3+2 \mathrm{H}_2 \mathrm{O}\)
The liberated ammonia gas is absorbed in a known volume (taken in excess) of a standard solution of sulphuric add.
⇒ \(2 \mathrm{NH}_3+\mathrm{H}_2 \mathrm{SO}_4 \longrightarrow\left(\mathrm{NH}_4\right)_2 \mathrm{SO}_4\)
The add that remains unused is estimated by titrating the solution with a standard solution of alkali.
The percentage of nitrogen in the organic compound can be calculated as follows.
- Let the mass of the organic compound = m g.
- Let the volume of the standard solution of added taking molarity M=V mL.
- Let the volume of unreacted acid (with ammonia) = V1 mL.
Let the volume of the standard solution of alkali of molarity M used for neutralising the unreacted acid = V2 mL.
The chemical reaction involved in the titration is
⇒ \(2 \mathrm{NaOH}+\mathrm{H}_2 \mathrm{SO}_4 \longrightarrow \mathrm{Na}_2 \mathrm{SO}_4+2 \mathrm{H}_2 \mathrm{O}\)
Thus at the endpoint,
⇒ \(n_1 M_1 V_1=n_2 M_2 V_2 \text {, }\)
where n1, M1 and V1 are the basicity, molarity and volume respectively of the acid used and;n2, M2 and V2 are the acidity, molarity and volume respectively of the base used. All these quantities are relevant only to the titration.
∴ \(\quad V_1=\frac{n_2 M_2 V_2}{n_1 M_1}=\frac{1 \times M \times V_2}{2 \times M}=\frac{V_2}{2}\)
or the volume of unreacted acid (with ammonia) \((V)=\frac{V_2}{2},\)
∴ The volume of acid reacted with NH3 = (V- V2/2) mL.
∴ Millimoles of NH3 that react with add- 2(V- V2/2)M
or moles of NH3 \(=\frac{2\left(V-V_2 / 2\right) M}{1000} .\)
Now moles of N in NH3 =1 x mole of NH3 \(=\frac{1 \times 2\left(V-V_2 / 2\right)}{1000} .\)
Mass of N \(=\frac{2\left(V-V_2 / 2\right)}{1000} \times 14 \mathrm{~g}\)
∴ Percentage of N in the organic compound
⇒ \(\begin{aligned}
& =\frac{2\left(V-V_2 / 2\right) M \times 14 \times 100}{1000 \times m} \\
& =\frac{14 \times M \times 2\left(V-V_2 / 2\right)}{m} .
\end{aligned}\)
Estimation Of Halogen
The quantity of a halogen in an organic compound is estimated by the Carius method. In this method, a known mass of the organic compound is taken in a hard glass-sealed tube (Carius tube) with fuming nitric acid in the presence of silver nitrate.
The Carius tube is then heated in a furnace. On heating carbon and hydrogen are oxidised to CO2 and H2O respectively while halogens form a precipitate of silver halide (AgX). The silver halide precipitate is filtered and washed with water and alcohol. It is then dried and weighed.
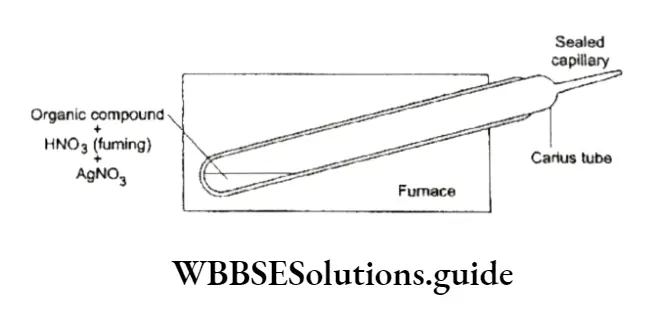
The percentage of the halogen is calculated as follows.
- Let the mass of the organic compound = m g.
- Mass of silver halide (AgX) obtained = m2 g.
108 + x parts by mass of AgX contains x parts by mass of halogen (x is the atomic mass of the halogen atom and 108 is the atomic mass of Ag).
(108 + x) g of AgX contains x g of X.
Therefore, mt g of AgX contains \(\frac{x}{108+x} \times m_1 g \text { of } \mathrm{X} .\)
Percentage of halogen X \(\mathrm{X}=\frac{x \times m_1}{(108+x)} \times \frac{100}{m} .\)
Estimation Of Sulphur
Sulphur is also estimated by the Carius method. A known mass of the organic compound is heated with fuming nitric acid in the Carius tube.
Sulphur is converted to sulphuric acid, which is precipitated by barium chloride as barium sulphate.
The precipitate of barium sulphate is filtered, washed, dried and weighed. From the mass of barium sulphate obtained, the percentage of sulphur can be calculated.
⇒ \(\begin{aligned}
& \mathrm{S}+\mathrm{H}_2 \mathrm{O}+3 \mathrm{O} \stackrel{\mathrm{HNO}_3}{\longrightarrow} \mathrm{H}_2 \mathrm{SO}_4 \\
& \mathrm{H}_2 \mathrm{SO}_4+\mathrm{BaCl}_2 \longrightarrow \mathrm{BaSO}_4 \downarrow+2 \mathrm{HCl}
\end{aligned}\)
Let the mass of the organic compound = m g.
Let the mass of the precipitate = m1 g.
233 g of BaS04 contains 32 g of S.
Therefore, m1 g of BaSQ4 contains \(\frac{32}{233} \times m_1 g \text { of } S\)
Percentage of S \(=\frac{32}{233} \times m_1 \times \frac{100}{m} .\)
Estimation Of Phosphorus
A known mass of the organic compound is heated with fuming nitric acid. The phosphorus in the organic compound is oxidised to phosphoric acid.
It is then treated with magnesia mixture (a solution containing magnesium chloride, ammonium chloride and a little ammonia).
A precipitate of magnesium ammonium phosphate (MgNH4P04) is obtained. It is filtered, washed, dried and then ignited to give magnesium pyrophosphate (Mg2P2O7), which is then weighed.
⇒ \(\mathrm{H}_3 \mathrm{PO}_4+\left(\mathrm{MgCl}_2+\mathrm{NH}_4 \mathrm{Cl}\right) \longrightarrow \mathrm{MgNH}_4 \mathrm{PO}_4
Magnesia mixture\)
Therefore, m1 g of Mg2P2O7 \(\frac{62}{222} \times m_1\) contains of phosphorus
Percentage of phosphorus \(=\frac{62}{222} \times \frac{m_1}{m} \times 100\)
Alternatively, phosphoric acid may be precipitated as ammonium phosphomolybdate, (NH4)3P04 -12Mo03, by adding ammonia and ammonium molybdate.
⇒ \(\mathrm{H}_3 \mathrm{PO}_4+\underset{\substack{\text { Ammonium } \\ \text { molybdate }}}{12\left(\mathrm{NH}_4\right)_2 \mathrm{MoO}_4}+21 \mathrm{HNO}_3 \longrightarrow \underset{\substack{\text { Ammonium } \\ \text { phosphomolybdate }}}{\left(\mathrm{NH}_4\right)_3 \mathrm{PO}_4 \cdot 12 \mathrm{MoO}_3 \downarrow}+21 \mathrm{NH}_4 \mathrm{NO}_3+12 \mathrm{H}_2 \mathrm{O}\)
Then the percentage of phosphorus will be \(=\frac{31 \times m_1 \times 100}{1877 \times m},\) where m mass 0f ammonium phosphomolybdate.
Estimation Of Oxygen
The percentage of oxygen is generally determined by subtracting the sum of percentages of all other elements from 100. However, it can be estimated directly by the following method.
A known amount of the organic compound is decomposed by heating in nitrogen gas.
The mixture of the gaseous product formed is passed over red hot coke when all the oxygen (present in the mixture) is converted into carbon monoxide.
The mixture is then passed through warm iodine pentoxide (I2O5) when carbon monoxide is oxidised to carbon dioxide and iodine is reduced.
⇒ \(\begin{gathered}
2 \mathrm{C}+\mathrm{O}_2 \stackrel{1373 \mathrm{~K}}{\longrightarrow} 2 \mathrm{CO} \\
\mathrm{I}_2 \mathrm{O}_5+5 \mathrm{CO} \longrightarrow 5 \mathrm{CO}_2+\mathrm{I}_2
\end{gathered}\)
The percentage of oxygen can be calculated from the amount of carbon dioxide or iodine produced.
Let the mass of the organic compound = mg.
Let the mass of carbon dioxide produced = m g.
44 g of CO2 contains 32 g of O.
Therefore, m1 g of CO2 contains \(\frac{32}{44} \times m_1 g \text { of } \mathrm{O}\)
Percentage of O \(=\frac{32 \times m_2 \times 100}{44 \times m} .\)
Earlier, for quantitative analysis large amounts of the pure compound (about 1 g) were required.
However, with the advances in the recent past, it is now possible to carry out the analysis with 3-4 mg of the substance. The accuracy in these estimations is ±0.03%. Automatic CHN elemental analysers are also available, which give results in a few minutes.
Example 1. Calculate the percentage of carbon, hydrogen and oxygen, if 02722g of an organic compound on complete combustion gives 05545g of carbon dioxide and 02227 g of water.
Solution:
Given
02722g of an organic compound on complete combustion gives 05545g of carbon dioxide and 02227 g of water.
Percentage of C =
⇒ \(\begin{aligned} & =\frac{12}{44} \times \frac{\text { mass of } \mathrm{CO}_2}{\text { mass of compound }} \times 100 \\
& =\frac{12}{44} \times \frac{0.5545}{0.2722} \times 100=54.5 .
\end{aligned}\)
Percentage of H=
⇒ \(\begin{aligned} & =\frac{2}{18} \times \frac{\text { mass of } \mathrm{H}_2 \mathrm{O}}{\text { mass of compound }} \times 100 \\ & =\frac{2}{18} \times \frac{0.2227}{0.2722} \times 100=9 \end{aligned}\)
Thus, the percentage ofO =100- (54.4 + 9) = 36.6.
Example 2. During the estimation of nitrogen by the Dumas method, 0.45 g of an organic compound gave 75mL of nitrogen, which was collected at 300 K and at 715 mmHg pressure. Calculate the percentage of nitrogen in the compound. (The vapour pressure of water at 300K is 15mmHg.)
Solution:
Given
During the estimation of nitrogen by the Dumas method, 0.45 g of an organic compound gave 75mL of nitrogen, which was collected at 300 K and at 715 mmHg pressure.
Vapour pressure of gas = (715- 15) mmHg = 700 mmHg.
Using the gas equation \(\frac{p_1 V_1}{t_1}=\frac{p_2 V_2}{t_2}\) the volume of nitrogen at 760 mmHg pressure and 0°C can be calculated as
⇒ \(V_2=\frac{700 \times 75 \times 273}{760 \times 300} \mathrm{~mL} .\)
22,400 mL of nitrogen at 760 mmHg pressure and (PC weighs 28 g.
Therefore, \(\frac{700 \times 75 \times 273}{760 \times 300}\)
mL of nitrogen at the same pressure and temperature weighs
⇒ \(\frac{28}{22,400} \times \frac{760 \times 300}{700 \times 75 \times 273}=0.0786 \mathrm{~g} .\)
Percentage of nitrogen \(=\frac{0.786}{0.45} \times 100=17.46\)
Example 3. During the estimation of nitrogen by the Kjeldahl method 0.5 g of an organic compound evolved ammonia, which was absorbed in 100 mL of \(\frac{M}{10} \mathrm{H}_2 \mathrm{SO}_4 \text {. }\)
The unused acid required 150 mL \(\frac{M}{10}\) NaOH solution. Calculate the percentage composition of nitrogen in the compound.
Solution:
Given
During the estimation of nitrogen by the Kjeldahl method 0.5 g of an organic compound evolved ammonia, which was absorbed in 100 mL of \(\frac{M}{10} \mathrm{H}_2 \mathrm{SO}_4 \text {. }\)
The unused acid required 150 mL \(\frac{M}{10}\) NaOH solution.
The chemical reaction taking place in the titration is
⇒ \(2 \mathrm{NaOH}+\mathrm{H}_2 \mathrm{SO}_4 \longrightarrow \mathrm{Na}_2 \mathrm{SO}_4+2 \mathrm{H}_2 \mathrm{O}\)
i.e., 2 moles of NaOH react with 1 mole of H2SO4.
M1V1=M2V2.
If the volume of the unused H2S04 is V1 then,
⇒ \(\begin{array}{rlrl}
& & V_1 \times \frac{1}{10} & =150 \times \frac{1}{10} \times \frac{1}{2} \\
\text { or } & V_1 & =75 \mathrm{~mL} .
\end{array}\)
Therefore, the volume of \(\frac{1}{10} \mathrm{MH}_2 \mathrm{SO}_4\) used by NH3 = 100- 75 = 25 mL.
Millimoles of H2S04 used by NH3 \(=25 \times \frac{1}{10}=2.5 .\)
Since \(2 \mathrm{NH}_3+\mathrm{H}_2 \mathrm{SO}_4 \longrightarrow\left(\mathrm{NH}_4\right)_2 \mathrm{SO}_4,\)
millimoles of NH3 = 2 x millimoles of H2S04
\(=2 \times 2.5=5.0 \text {. }\)Mass of NH3 formed = moles x molar mass
\(=5.0 \times 10^{-3} \times 17 \text {. }\)Mass of N \(=\frac{5.0 \times 10^{-3} \times 17 \times 14}{17} \mathrm{~g}\)
\(=5.0 \times 10^{-10} \times 14 \mathrm{~g}\) \(\text { Percentage of } \mathrm{N}=\frac{1 .}{1000} \times 5.0 \times \frac{100}{0.5}=14 .\)Example 4. During bromine estimation by the Carius method, 0.165 g of a compound gave 0.132 g of AgBr. Find the percentage of bromine in the compound.
Solution:
Given
During bromine estimation by the Carius method, 0.165 g of a compound gave 0.132 g of AgBr.
Molecular mass of AgBr = 188
188 g of AgBr contains 80 g of bromine.
0.132 g of AgBr contains \(\frac{80}{188} \times 0.132 \mathrm{~g} \text { of bromine. }\)
Percentage of bromine in the compound \(=\frac{80 \times 0.132}{188} \times \frac{100}{0.165}=34 .\)
Example 5. During sulphur estimation by the Carius method, 0.434 g of an organic compound gave 0640 g of BaSO, Calculate the percentage of sulphur in the compound.
Solution:
Given
During sulphur estimation by the Carius method, 0.434 g of an organic compound gave 0640 g of BaSO
233 g of BaS04 contains 32 g of sulphur.
0.640 g of BaS04 contains \(\frac{32}{233} \times 0.640 \mathrm{~g}\) of sulphur.
Percentage of S \(=\frac{32}{233} \times \frac{0.640 \times 100}{0.434}=20.27\)
Organic Chemistry—Some Basic Principles And Techniques Multiple Choice Questions
Question 1. Which of the following has the least C—C bond length?
- Ethane
- Ethene
- Ethyne
- All are equal
Answer: 3. Ethyne
Question 2. How many tertiary hydrogen atoms are present in
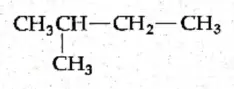
- One
- Two
- Three
- Four
Answer: 1. One
Question 3. The compounds butene-1 and isobutene exhibit
- Chain isomerism
- Position isomerism
- Functional isomerism
- Metamerism
Answer: 1. Chain isomerism
Question 4. Which of the following has maximum -I effect?
- OH
- COOH
- Br
- NQ2
Answer: 4. NQ2
Question 5. Which of the carbon atoms numbered will have a maximum 6+ charge in the given compound?
⇒ \(\stackrel{3}{\mathrm{C}} \mathrm{H}_3-\stackrel{2}{\mathrm{C}} \mathrm{H}_2-\stackrel{1}{\mathrm{C}} \mathrm{H}_2-\mathrm{Cl}\)
- Carbon 1
- Carbon 2
- Carbon 3
- All will have equal 5+ charges.
Answer: 1. Carbon 1
Question 6. Which of the following carbocations is the most stable?
- \(\mathrm{CH}_3 \mathrm{CH}_2 \stackrel{+}{\mathrm{C}} \mathrm{H}_2\)
- \(\mathrm{CH}_2=\mathrm{CH}-\stackrel{+}{\mathrm{C}} \mathrm{H}_2\)
- \(\mathrm{C}_6 \mathrm{H}_5 \stackrel{+}{\mathrm{C}} \mathrm{H}_2\)
- All are Equally Stable
Answer: 3. \(\mathrm{C}_6 \mathrm{H}_5 \stackrel{+}{\mathrm{C}} \mathrm{H}_2\)
Question 7. Which of the following free radicals is the most stable?
- Methyl
- Ethyl
- Isopropyl
- Tertiary butyl
Answer: 4. Tertiary butyl
Question 8. Which of the following is/are electrophilic?
- NO2+
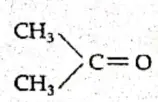
- C2H5Cl
- All the above
Answer: 4. All the above
Question 9. In which of the following reactions has the hybridised state of a carbon atom changed?
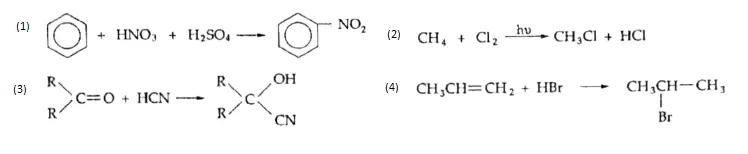
Answer: 3 and 4
Question 10. The IUPAC name of
- Butanal
- Butan-l-al
- Butane-l-carboxaldehyde
- Pentan-l-al
Answer: 4. Pentan-l-al
Question 11. The IUPAC name of CH3CH=CH—C=CH is
- 4-Pentyn-2-ene
- l-Pentyn-3-ene
- 3-Penten-l-yne
- 2-Penten-4-yne
Answer: 3. 3-Penten-l-yne
Question 12. Identify the molecule/ion in which the inductive, resonance and hyperconjugative effects are operative.
- (CH3)3C
- CH2=CH—CH=0
- (CH3)3C—CH2
- CH3CH=CH—CH=0
Answer: 4. CH3CH=CH—CH=0
Qualitative 13. The smallest alkane with a 3° carbon is
- Neopentane
- Isopentane
- Isohexane
- Isobutane
Answer: 4. Isobutane
Question 14. Sublimation can be used for the purification of
- Glucose
- Acetamide
- Naphthalene
- Formic acid
Answer: 3. Naphthalene
Question 15. Glycerol boils at 536 K and decomposes at its bp. It can be purified by
- Fractional distillation
- Steam distillation
- Sublimation
- Distillation under reduced pressure
Answer: 4. Distillation under reduced pressure
Question 16. A mixture of benzene and toluene can be separated by
- Steam distillation
- Fractional distillation
- Vacuum distillation
- Simple distillation
Answer: 2. Fractional distillation
Question 17. Which of the following will respond positively to Lassaigne’s test for nitrogen?
- Nitrobenzene
- Acetamide
- Urea
- All the Above
Answer: 4. All the Above
Question 18. In Lassaigne’s test, when both N and S are present, a blood red colour is obtained. This is due to the formation of
- Ferriferrocyanide
- Ferrithionate
- Sodium thiocyanate
- Ferricyanide
Answer: 2. Ferrithionate
Question 19. A mixture of acetone (b.p. 329 K) and methyl alcohol (b.p. 338 K) can be separated by
- Simple distillation
- Distillation under reduced pressure
- Steam distillation
- Fractional distillation
Answer: 4. Fractional distillation
Question 20. In column chromatography, the separation of a mixture of two substances depends on their
- Different solubilities
- Different melting points
- Different specific gravities
- Differential adsorption
Answer: 4. Differential adsorption
Question 21. A substance which is insoluble in water and possesses a vapour pressure of 10-15 mmHg at 373 K can be purified by
- Crystallisation
- Distillation
- Steam distillation
- Sublimation
Answer: 3. Steam distillation
Question 22. In steam distillation, the total pressure of the organic substance and water vapour becomes
- Equal to the atmospheric pressure
- More than the atmospheric pressure
- Less than the atmospheric pressure
- There is no fixed relationship
Answer: 1. Equal to the atmospheric pressure
Question 23. 0.4 g of an organic compound on complete combustion gives 0.18 g of water. What will be the percentage of hydrogen in the compound?
- 10
- 15
- 5
- 2.5
Answer: 3. 5
Question 24. Which of the following compounds will give a blood-red colouration when nitrogen is present?
- M-nitrobenzoic add
- P-toluidine
- Urea
- M-nitrobenzene sulphonic add
Answer: 4. M-nitrobenzene sulphonic add
Question 25. In the Kjeldahl method, the nitrogen present in an organic compound is estimated by measuring the amount of
- N2
- NO2
- NH3
- (NH4)2SO4
Answer: 3. NH3
Question 26. Which of the following reagents is added to the sodium extract of an organic compound to detect die presence of sulphur in the compound?
- Sodium nitroprusside
- Ammonium molybdate
- Ammonium phosphomolybdate
- Sodium hexacyanoferrate
Answer: 1. Sodium nitroprusside
Question 27. In the sodium fusion test of organic compounds, the nitrogen of an organic compound is converted to
- Sodium nitrate
- Sodium nitrite
- Sodium amide
- Sodium cyanide
Answer: 4. Sodium cyanide
Question 28. Carbon and hydrogen present in an organic compound are estimated by
- The dumas method
- The various method
- The Liebig method
- None of these
Answer: 3. The lie big method
