Fractional Oxidation Number
So far we have discussed whole-number oxidation states of elements. You may wonder how oxidation numbers can be fractional since electrons are never shared or transferred in fractions.
But sometimes we come across fractional oxidation states of elements in certain compounds. For example, the oxidation numbers of both Pb in Fb04 and Fe in Fe304 are + 8.
In these cases the oxidation number calculated is the average oxidation number of all the like atoms in the molecule, e.g., in Fe304 two Fe atoms have the oxidation number +3 and one has the oxidation number +2, so the average oxidation number for each Fe atom is +%, which is also the case for Pb304.
Fe304 and Pb304 are stoichiometric compounds and are referred to as ‘mixed oxides’.
They are better formulated ns FeO -Fe203 and 2PbO -PbOj respectively and the oxidation numbers of Fe arc +2 and +3 while those of Pb are +2 and +4 respectively.
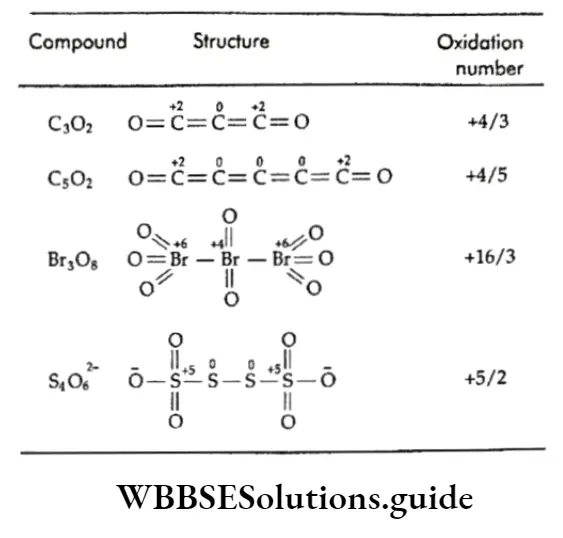
Other such cases of fractional oxidation numbers are observed in C302, C502, Br30g, and S4O2-6.
“Oxidation number, definition, calculation and examples, explained”
The structures of such compounds show the different oxidation states of atoms, which depend on the bonding of the atoms.
The structures of the compounds in which the oxidation number of an element is fractional.
Thus, the fractional oxidation numbers displayed by elements in certain compounds are merely the respective average oxidation numbers of all the atoms with different oxidation states in those compounds.
Fractional oxidation numbers are not a reality.
| Class 11 Biology | Class 11 Chemistry |
| Class 11 Chemistry | Class 11 Physics |
| Class 11 Biology MCQs | Class 11 Physics MCQs |
| Class 11 Biology | Class 11 Physics Notes |
They signify the fact that in certain compounds the atoms of the same element can be bonded to the other elements in more than one way (so that the same element exhibits more than one oxidation state) to achieve stability.
Redox Reactions In Terms Of Oxidation Number
The transfer of electrons from one species to the other in a redox reaction leads to a change in their oxidation numbers.
Therefore, a redox reaction can also be defined in terms of oxidation number.
A redox reaction is a reaction that involves a change in the oxidation number of the interacting species.
Similarly, the idea of oxidation number change can also be applied to define oxidation, reduction, oxidant (oxidizing agent), and reductant (reducing agent).
Thus, when the oxidation number of an atom or a group of atoms increases, it undergoes oxidation. Contrary to this, when the oxidation number decreases, reduction takes place.
A substance acts as an oxidizing agent if the oxidation number of one (or more) of its atoms decreases and as a reducing agent if the oxidation number of one (or more) of its atoms increases.
In other words, a species itself undergoing oxidation is a reducing agent and vice versa.
Consider the following redox reactions.
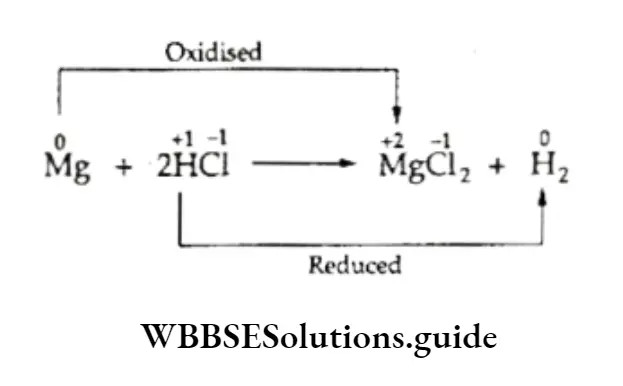
In this reaction the oxidation number of magnesium changes from 0 to +2 and that of hydrogen from +1 to 0.
The oxidation number of chlorine remains unchanged. Thus, magnesium is oxidized and hydrogen is reduced.
“Oxidation number, definition, calculation and examples, step by step”
In the reaction, magnesium acts as a reductant and reduces hydrogen. Hydrogen, on the other hand, acts as an oxidant and oxidizes magnesium.
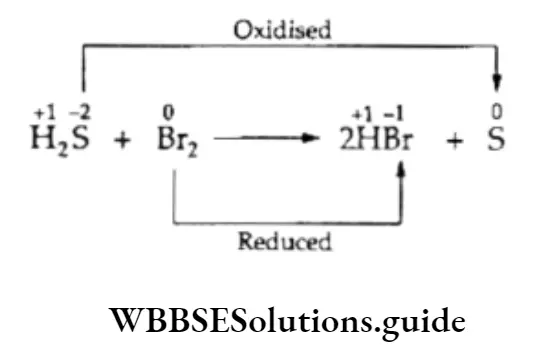
Here the oxidation number of sulfur increases from -2 to 0, while that of bromine decreases from 0 to -1.
This indicates that sulfur (the reductant) is oxidized and bromine (the oxidant) is reduced. The oxidation number of hydrogen remains unchanged.
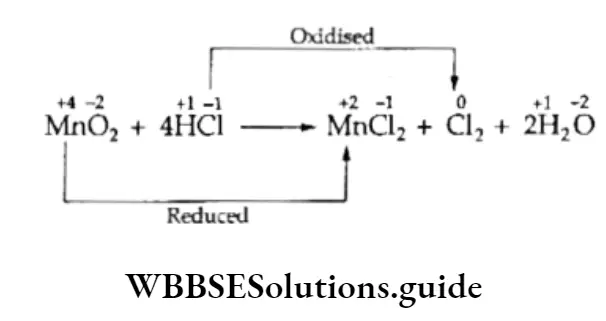
In this reaction, as you can see, manganese is reduced and chloride is oxidized. Here manganese, being the oxidant, oxidizes chlorine, which is the reductant.
Example 1.
Identify the oxidant and reductant in the following reactions:
⇒ \(2 \mathrm{NaBr}+\mathrm{Cl}_2 \longrightarrow 2 \mathrm{NaCl}+\mathrm{Br}_2\)
⇒ \(\mathrm{MnO}_4^{-}+8 \mathrm{H}^{+}+5 \mathrm{Fe}^{2+} \longrightarrow \mathrm{Mn}^{2+}+5 \mathrm{Fe}^{3+}+4 \mathrm{H}_2 \mathrm{O}\)
⇒ \(\mathrm{CH}_4+\mathrm{Cl}_2 \longrightarrow \mathrm{CCl}_4+4 \mathrm{HCl}\)
Solution:
To identify the oxidant and reductant in each of the reactions, we should indicate the oxidation number of each atom in order to identify the atoms whose oxidation numbers change.
⇒ \(2 \stackrel{1}{\mathrm{~N} a} \stackrel{-1}{\mathrm{Br}}^{-1} \stackrel{0}{\mathrm{C}} \mathrm{l}_2 \longrightarrow 2 \stackrel{+1}{\mathrm{Na}} \stackrel{-1}{\mathrm{Cl}}+\stackrel{0}{\mathrm{Br}_2}\)
The oxidation number of Na does not change. The oxidation number of Br changes from -1 to 0, therefore it is oxidized and is the reductant. The oxidation number of Cl changes from 0 to -1. So it is reduced and is the oxidant.
⇒ \(\mathrm{MnO}_4^{-2}+8 \stackrel{+}{+}+5 \mathrm{~F}^{2+} \longrightarrow \mathrm{Mn}^{2+}+5 \mathrm{Fe}^{3+}+4 \mathrm{H}_2 \mathrm{O}^{-2}\)
The oxidation numbers of H and O remain unchanged. The oxidation number of Mn changes from +7 to +2. Thus, it is reduced and is the oxidant. The oxidation number of Fe increases from +2 to +3. It is oxidized and is thus the reductant.
⇒ \(\stackrel{-4}{\mathrm{C}} \stackrel{+1}{\mathrm{H}}_4+4 \stackrel{0}{\mathrm{C}} \mathrm{Cl}_2 \longrightarrow \stackrel{+4}{\mathrm{C}} \stackrel{-1}{\mathrm{C}} \mathrm{l}_4+\stackrel{+1}{4} \stackrel{-1}{\mathrm{Cl}}^{-1}\)
The oxidation number of H does not change. The oxidation number of C changes from -4 to +4. Thus, it is oxidized and is the reductant. Cl is the oxidant as it is reduced, as is revealed by the decrease in its oxidation number from 0 to -1.
Redox Reactions Oxidation Number
Redox reactions basically involve the transfer of electrons from one reactant to another. When such a reaction involves ionic species, the transfer of electrons is obvious, as it is in the following case.
⇒ \(\mathrm{Zn}(\mathrm{s})+\mathrm{Cu}^{2+}(\mathrm{aq}) \longrightarrow \mathrm{Zn}^{2+}(\mathrm{aq})+\mathrm{Cu}(\mathrm{s})\)
However, the exchange of electrons is not very obvious when redox reactions occur between covalent molecules, as in the following reaction.
⇒ \(\mathrm{H}_2(\mathrm{~g})+\mathrm{Cl}_2(\mathrm{~g}) \longrightarrow 2 \mathrm{HCl}(\mathrm{g})\)
In this reaction the reactants and products are all covalent, i.e., the bond formed between the atoms involves the sharing of electrons.
However, chlorine has a higher electronegativity than hydrogen.
“Oxidation number, definition, calculation and examples, for beginners”
Therefore, in hydrogen chloride the bond pair is closer to chlorine, resulting in the partial transfer of electronic charge from hydrogen to chlorine as shown below.
⇒ \(\stackrel{8+}{\mathrm{H}}-\stackrel{8}{\mathrm{Cl}}^{-}\)
Due to this charge separation, we may say that chlorine acts as an oxidant while hydrogen acts as a reductant. It may be pointed out that the traditional definition of redox reactions very easily explains this reaction.
Chlorine is reduced to hydrogen chloride by gaining hydrogen. Hydrogen, thus, is a reductant.
In order to identify the oxidant and reductant readily and overcome the problem of determining the number of electrons transferred during any redox reaction, the concept of oxidation number or oxidation state was introduced.
The oxidation number of an element may be defined as the charge that its atom has in a compound.
In covalent compounds, the effective charge on the atoms is not very apparent.
Therefore, it is useful to assign a charge to each element by a set of rules agreed upon by scientists.
This assigned charge is called the oxidation number or oxidation state, and may or may not represent the actual charge.
Rules For Assigning Oxidation Number
There are certain universally accepted rules for determining the oxidation number of an element in the combined state.
These are listed as follows.
- The oxidation number of an element in the uncombined or free state is zero. Thus, the oxidation numbers of the respective elements in H2, He, Cl 2, S 8, C, and P4 are zero.
- As the atoms do not differ in electronegativity, in such cases, the bond pair is equally shared and no atom has residual charge.
- The oxidation number of an element in a monoatomic ion is the same as the charge on it. For example, the oxidation numbers of Na++, Ca2+, Al3+, I+, S2- and N3- ions are +1, +2, +3, -1, -2,-3 respectively.
- The algebraic sum of oxidation numbers of all the atoms in a neutral molecule is zero. In the case of an ionic species, the algebraic sum of the oxidation numbers of the atoms in the ion must be equal to the net charge on the ion.
- For instance, the algebraic sum of the oxidation numbers of carbon and oxygen inCO3- must be 2.
- Hydrogen is assigned the oxidation number of +1 in most of its compounds. However, when it combines with active metals to form hydrides like LiH, NaH, and MgHj, it has an oxidation number of 1.
- The oxidation number of oxygen in most of its compounds is -2. However, in peroxides like H2O2, Na2O2, and BaO2 it is -1. In oxygen difluoride (OF2) and dioxygen difluoride (02F2) the oxidation number of oxygen is +2 and +1 respectively because fluorine, being the most electronegative element in the periodic table, is always assigned the oxidation number of 1.
- The halogens other than fluorine have an oxidation number of -1 except when they combine with a more electronegative halogen or oxygen, e.g., in C120 and C1F3, chlorine has an oxidation number of +1 and +3 respectively. In IF7 the oxidation number of iodine is +7.
- The oxidation number of alkali metals (Li, Na, K, Rb, Cs) is always +1 and that of alkaline earth metals (Be, Mg, Ca, Sr, Ba) is +2.
- When two different elements combine to form a compound, the more electronegative element has a negative oxidation number, while the less electronegative element has a positive oxidation number. For instance, the oxidation numbers of nitrogen in NH3 and NC13 are 3 and +3 respectively.
Example. Calculate the oxidation numbers of the following:
1. C in CO2
- Let the oxidation number of C in CO2 be x.
- The oxidation number of each O atom is -2.
- The sum of the oxidation numbers of all the atoms in CO2 is zero.
- ∴x-4 = 0orx =4
- Thus, the oxidation number of C in CO2 is +4.
2. Mn in KMnO4
- Let the oxidation number of Mn in KMnO4 be x.
- The oxidation number of each O atom is -2 and that of K is +1.
- The sum of the oxidation numbers of all the atoms in KMnO4 is zero.
- ∴l+x-(2×4) = 0 or x = 7
- Thus, the oxidation number of Mn in KMnO4 is +7.
3. Pb in Pb02
- Let the oxidation number of Pb in PbO2 be x.
- The oxidation number of each O atom is -2.
- The sum of the oxidation numbers of all the atoms in Pb02 is zero.
- ∴x-(2×2) = 0 or x = 4.
- Thus, the oxidation number of Pb in Pb02 is +4.
4. CI1KCIO4
- Let the oxidation number of Cl in CIO4– be x.
- The oxidation number of each O atom is -2.
- The sum of the oxidation numbers of all the atoms in CIO-4 is -1.
- ∴x-(2×4)=-l or x =7.
- Thus, the oxidation number of Cl in CIO-4 is +7
5. NinNH4+
- Let the oxidation number of N in NH4 be x.
- The oxidation number of each H atom is +1 and the sum of the oxidation numbers of all the atoms in
- NH+4 is also+1.
- ∴ x + (lx4) =l or X =-3.
- Thus, the oxidation number of N in NH4 is -3.
6. Cr in Cr2Oj
- Let the oxidation number of each Cr atom in Cr2OV be x.
- The oxidation number of each O atom is -2.
- The sum of the oxidation numbers of all the atoms in Cr2Of~ is -2.
- ∴xx2 -1-(-2×7) = -2
- or 2x-14 = -2; 2x =12 or x = 6.
- Thus, the oxidation number of Cr in Cr2O2-7 is +6.
“Oxidation number, definition, calculation and examples, PDF notes”
Variable Oxidation Number
Variable Oxidation Number:
By now it should be clear to you that the oxidation number of an element is not necessarily the same in all compounds, it varies.
For instance, the oxidation numbers of C in CCI4/ CH4 and CH2C12 are +4, -4, and 0 respectively. The oxidation numbers of O in OF2 and C120 are +2 and -2 respectively.
This is because apart from the highly electropositive metals of groups and 2 which have oxidation numbers +1 and +2 respectively and the most electronegative element—fluorine which has the oxidation number of, the oxidation numbers of most of the remaining elements vary as they depend on the other combining elements.
Transition metals exhibit a maximum variation in the oxidation state as compared to the other groups of the periodic table.
The oxidation numbers of Fe in FeSO4 and FeCl3 are +2 and +3 respectively. Manganese shows a number of different oxidation states— +2 in MnClÿ +3 in Mn(OH)3, +4 in MnOÿ +6 in K2 MnO4 and +7 in KMnO4. The oxidation states of the elements in the 3d series of transition elements.
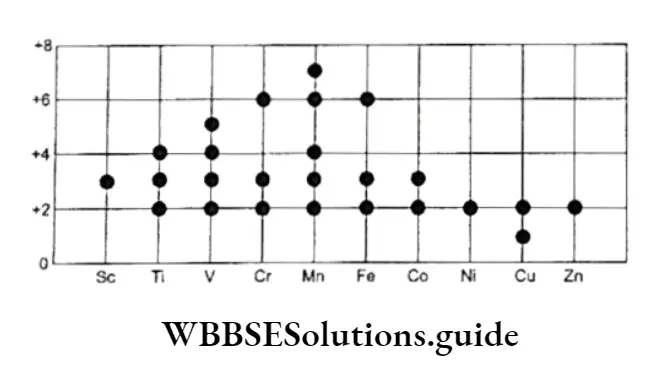
The compounds of the same element in different oxidation states often differ in color. The highest oxidation state is generally in the form of oxonian, MnO4, CrO4, etc.
These are excellent oxidizing agents. Many elements of the p block also display variable oxidation states. For example, it shows an oxidation number of +2 in SnCl2 and one of +4 in
SnCl4
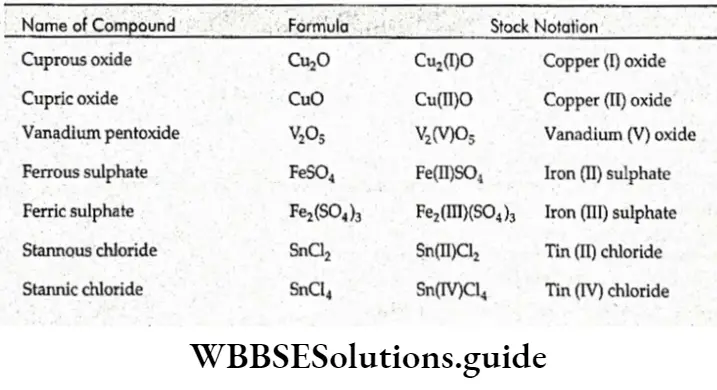
Oxidation Number And Nomenclature
The names of compounds initially did not have much to do with their compositions.
Compounds were named on the basis of some characteristic property like color or the source from which they were derived. Thus CuSO4 -5H2O and FeSO4 >7H2O were called blue vitriol and green vitriol on the basis of their color.
They were both derived from vitriolic acid or oil of vitriol, the trivial name for sulphuric acid.
As science progressed and more and more compounds were identified, it was necessary to follow a systematic nomenclature based on the chemical composition of compounds. Thus, FeSO4 was named ferrous sulfate.
According to this system, binary compounds are named by writing the electropositive element followed by the electronegative element and adding the suffix ide to the latter, e.g., sodium chloride (NaCl).
In the case of metals exhibiting variable oxidation states, the lower oxidation state has the suffix ous and the higher oxidation state has the suffix is, e.g., Cu20 is a cuprous oxide (copper has an oxidation state of 1) and CuO is a cupric oxide (the oxidation state of Cu is 2).
You are familiar with this system of nomenclature so we will not go into further details of it.
“Oxidation number, definition, calculation and examples, chemistry guide”
Stock Notation
The modem system of naming compounds of metals exhibiting variable oxidation states is called Stock notation after the scientist, Albert Stock, who devised it.
The tire oxidation state of the metal is indicated in Roman numerals, enclosed in brackets, and is written after the name or symbol of the metal.