The P-Block Elements
The elements in which the last electron enters the p orbital constitute the p-block elements. There are three degenerate p orbitals in a shell and each can accommodate a maximum of two electrons.
When tyre energy levels are filled in the sequence to build up elements, the number of electrons present in the p orbitals varies from one to six, and hence there are six groups of p-block elements.
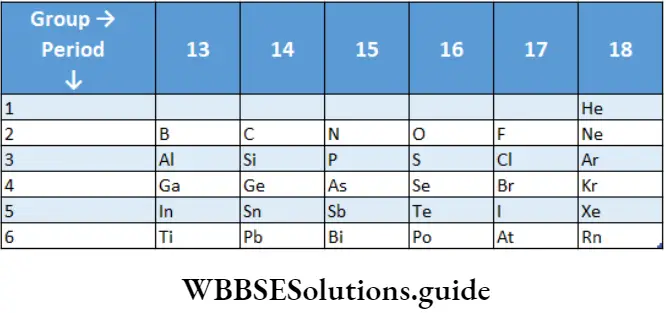
General Trends
The valence-shell electronic configuration of p-block elements is Ns2np1-6 except for that of He, which is Is2. The size and metallic character of the elements increase on moving down the group and decrease on moving from left to right across a period.
Unlike s-block 1s2 elements, which are all metallic in nature, the p-block elements comprise metals, metalloids and nonmetals; the lighter members are nonmetals while the heavier ones are predominantly metallic.
The chemistry of p-block elements is complex as both metals and nonmetals are to be dealt with in contrast to s-block elements, which are all metals. In addition, there are some elements whose properties are intermediate between those of the metals and the nonmetals.
These are referred to as metalloids, e.g., arsenic and antimony. It may be emphasised that except in the p block, nonmetals and metalloids do not occur anywhere in the periodic table.
| Class 11 Biology | Class 11 Chemistry |
| Class 11 Chemistry | Class 11 Physics |
| Class 11 Biology MCQs | Class 11 Physics MCQs |
| Class 11 Biology | Class 11 Physics Notes |
Metals generally have low ionisation enthalpies and low electronegativities and form cations whereas nonmetals form anions. The p-block elements form both ionic and covalent compounds.
The ionisation enthalpy, electronegativity and oxidising power decrease down the group and increase across a period in the p block. A striking behaviour of the p-block elements is the display of two oxidation states—higher and lower.
The higher oxidation state is equal to the group number minus 10, i.e., the total number of s and p electrons in the valence shell and the lower one is two units less than the higher oxidation state, i.e., equal to the number of p electrons of the valence shell.
The stability of the lower oxidation state increases on moving down the group.
The p-block elements exhibit a higher oxidation state when both the ns and up electrons of the valence shell are used in bond formation. On the other hand, the lower oxidation state is observed only when the tup electron(s) of the valence shell participate in bonding.
This reluctance of the outermost s-orbital electron pair to participate in bond formation is called the inert-pair effect. This can be explained in terms of energy.
On the one hand, energy is needed to uncouple the valence-shell-paired s electrons and on the other, it is released when the electrons participate in bond formation.
If the bond energy is high enough to compensate for the amount needed to uncouple, the s electrons participate in bond formation.
“p-Block elements, definition, properties, uses, and examples”
On moving down the groups, the bond energy decreases and the valence-shells electrons remain paired and the lower oxidation state becomes more stable.
In contrast to s-block elements, which display only positive oxidation states, p-block elements display both positive and negative oxidation states.
As the electronegativity of the elements increases tyre negative oxidation states become more stable.
This will become clearer to you when we discuss specific examples while studying the different groups separately.
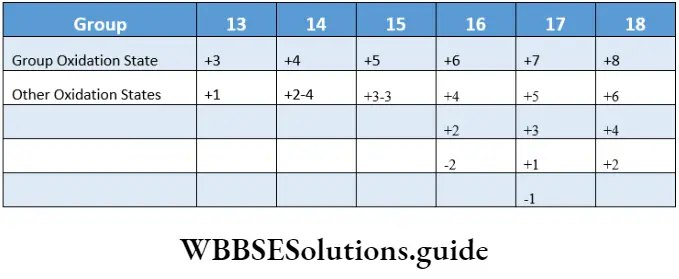
A regular gradation in properties is not noted in the p-block elements (unlike the s-block elements) as we come across both metals and nonmetals.
The display of multiple oxidation states adds to the complexity. The first member behaves differently from the other members of the group.
We have observed this in s-block elements also —lithium and beryllium, due to their small size, show anomalous behaviour.
Another important characteristic of the p-block elements is their ability, from the third period onwards, to utilise the vacant d orbitals and hence expand their covalence.
Boron (second period) has a maximum covalence of four (one 2s and three 2p orbitals), e.g., BF4-, but aluminium (third period) can utilise 3d orbitals in addition to 3s and 3p and form species like [AlF6]3- where the covalence is six.
In the third period elements of p-block, the general valence-shell electronic configuration is 3s2 3pn.
The atoms of the elements have vacant 3D orbitals lying between the 3p and 4s energy levels. Using these orbitals the elements expand their covalence above four.
Another unique feature of p-block elements is the ability of the first member of each group to form pπ-pπ multiple bonds with itself or with the other elements in the same row.
Thus we have innumerable species containing C=C and C=C bonds, but hardly any displaying Si=Si bonds. Elemental nitrogen and oxygen contain triple and double bonds respectively (N=N, 0=0) but for the lower members such linkages are not observed.
There are also many compounds which have C=0, C=N, and N=0 bonds. A pπ-pπ bond is formed by the sideways overlap of p orbitals.
For maximum overlap to occur, the orbitals should be small and of comparable size. For the heavier members, the orbitals become large and diffuse and the effective overlap does not occur.
This does not mean that the heavier (or lower) members do not form rr-bonds.
In SO42– and PO43– there is multiple bonding between sulphur and oxygen, and phosphorus and oxygen respectively. These bonds are formed involving p orbitals of oxygen and d orbitals of sulphur and phosphorus and are referred to as pπ-dπ bonds.
The d orbitals are higher in energy than the p orbitals, so they contribute less to the stability of the molecule than the pn-pn bond. You will study more about this in higher classes.
We will now discuss in detail Groups 13 and 14 with special emphasis on boron, aluminium, carbon and some important compounds.
Group 13 Elements
The elements in this group are boron, aluminium, gallium, indium and thallium. Boron is a nonmetal while the others are fairly reactive metals. The metallic character increases down the group. The general valence-shell electronic configuration is ns2 np1.
Occurrence
Boron is a rare element and occurs to the extent of 0.0001% by mass in the earth’s crust. There are two isotopes of boron — 10 B and 11 B —abundant in the ratio 1:4.
Boron occurs principally in the earth’s crust as boric acid (H3B03) and as borates, such as borax (Na2B4O7.10H2O), kemite (Na2B4O7.4H2O) and colemanite, (Ca2B6O11 -5H2O).
Aluminium is the most abundant metal (8.13%) in the earth’s crust and the third-most abundant element (after oxygen and silicon).
The most important ore of aluminium is bauxite, Al2O3 .xH2O (varies between and 3). Bauxite is a mixture of aluminium oxide and hydroxide.
The other ores of aluminium are cryolite ( Na3 A1F6 ) and corundum (A12O3). Aluminium is widely present in aluminosilicate rocks like feldspars and micas. These rocks form clay minerals on weathering.
Aluminium, however, cannot be extracted from feldspars, micas and clay minerals. Gallium, indium and thallium are much less abundant and mainly occur as sulphide ores.
The main ore of gallium is germanite (a mixed sulphide of zinc, copper, gallium, germanium and arsenic).
Indium and thallium are present in traces in the sulphide ores of zinc and lead respectively.
The important physical properties of Group 13 elements
Electronic Configuration
The general outer electronic configuration of the elements is ns2npx. The inner cores of boron and aluminium have noble-gas configuration while for the other members, there are ten d electrons present in addition to the noble-gas configuration.
The d electrons affect size, ionisation enthalpy, electronegativity and chemical properties of the elements.
Atomic and ionic radii
The atomic radii of Group 13 elements are less than those of the corresponding s-block elements.
The atomic size does not increase regularly, as expected, on descending the group. The atomic radius of A1 (143 pm) is greater than that of B (85 pm) which is the expected trend.
However, the atomic radius of Ga (135 pm) is less than that of Al. This is because B and A1 follow the s-block elements whereas Ga follows immediately after the d-block elements.
Therefore, the inner core of Ga contains ten d electrons, which do not shield the nuclear charge efficiently.
(Shielding of electrons is in the order: s > p > d > f.) Therefore, the effective nuclear charge of Ga is more than that of Al so the outer electrons are attracted towards the nucleus and the size is smaller than expected.
Similarly, the inclusion of fourteen, poorly shielding 4f electrons affects the size of Tl.
Ionisation enthalpy
There is a decrease in ionisation enthalpies of the elements on moving down the group, but the decrease is not regular. The ionisation enthalpies of Ga and Tl are higher than expected due to the higher effective nuclear charge as a result of poor screening by d and f electrons.
The second and third ionisation enthalpies of Group 13 elements are very high as compared to their first ionisation enthalpies, which are even lower than those of the corresponding Group 2 elements.
This is because the first electron to be removed from Group 13 elements is an unpaired p electron whereas that in Group 2 elements is a paired s electron.
And we already know that the s orbital is more penetrating than the p orbital. Since the sum of the first three ionisation enthalpies is immense, it follows that a lot of energy is needed to form tri positive cations (M3+) B forms covalent compounds, as the sum of its first three ionisation enthalpies is very high.
Many simple compounds of Al and Ga are covalent when anhydrous. However, in solution, the large amount of hydration enthalpy evolved compensates for the high ionisation enthalpy and the compounds formed by Group 13 elements are ionic.
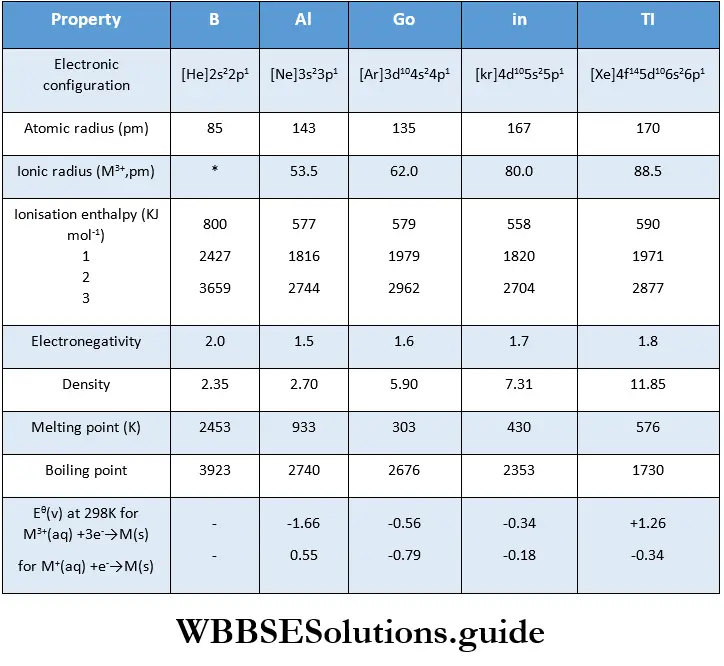
Electronegativity
Due to irregularities in the atomic size of the elements, a regular variation in electronegativity is not noted.
Electronegativity first decreases from B to Al and then increases to a small extent. This is due to the differences in the atomic structures of the elements.
Oxidation states
The common oxidation states are +3 and +1. As already discussed, the lower oxidation state arises due to the inert pair effect.
The stability of the +1 oxidation state increases down the group because ns electrons are prevented from participating in bond formation due to the increased effective nuclear charge.
Thus for B, Al and Ga, the +3 oxidation state is stable (the +1 oxidation state for Ga is reducing), for both are comparable instability, while for Tl the predominant oxidation state is +1 (+3 state is oxidising). Ga appears to be divalent in GaCl2, but actually, GaCl2 is represented as Ga+[GaCl4 ]– which contains Ga(I) and Ga(III).
Physical properties
- The precise determination of the physical properties of elemental boron is hampered due to two difficulties— the existence of a large number of allotropic forms and contamination by irremovable impurities.
- It is a black, extremely hard, refractory solid with a high melting point, low density and low electrical conductivity.
- The other elements are low-melting, rather soft metals and are good conductors of electricity.
- Gallium has an unusually low melting point and contracts on melting.
Chemical reactivity
Tyre elements of Group 13 are less reactive than those of the s block.
They generally form covalent compounds due to their small size, a large sum of the first three ionisation enthalpies and higher electronegativity values than those of the corresponding Group 1 and Group 2 elements.
Reaction with air
All elements of Group 13 react with oxygen at high temperatures to form oxides—M2O3
⇒ \(4 \mathrm{M}(\mathrm{s})+3 \mathrm{O}_2(\mathrm{~g}) \rightarrow 2 \mathrm{M}_2 \mathrm{O}_3(\mathrm{~s})\)
Thallium also forms T12O. A very thin layer of oxide forms on aluminium which prevents further reaction.
The basic character of oxides increases down the group—B2O3 is acidic, A12O3 and Ga2O3 are amphoteric whereas ln2O3 and T12O3 are basic.
T120 dissolves in water to form TlOH, which is as strong a base as KOH. Only boron and aluminium react with nitrogen at a very high temperature to form nitrides.
⇒ \(2 \mathrm{Al}+\mathrm{N}_2 \rightarrow 2 \mathrm{AlN}\)
Reaction with halogens
Trihalides of all elements are known. In addition, thallium also forms monohalides. The trihalides are electron-deficient compounds as the central atom has only six electrons (3 valence electrons and 3 from halogens, e.g., BC13).
Such compounds have a strong tendency to accept a pair of electrons and complete the octet, and thus act as Lewis acids.
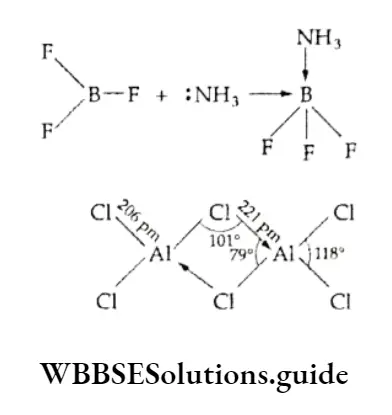
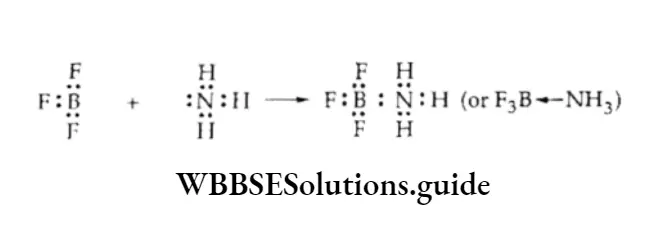
On moving down the group, the tendency to behave as Lewis acid decreases. BC13 or BF3 readily accepts a pair of electrons from Lewis bases like ammonia to form BF3-NH3.
Aluminium chloride remedies the electron deficiency by forming a dimer (a halogen-bridged molecule) in which each aluminium atom completes its octet by accepting an electron pair from chlorine.
The halides are covalent and get hydrolysed in water, forming four coordinate species like [M(OH)4]–.
These are tetrahedral in structure where the central element, M, is sp3 hybridised. The halides also tend to form octahedral hydrated species like [A1(H2O)6]3+, which is formed when aluminium chloride is dissolved in acidified water. In such species, the central metal is sp3d2 hybridised. Since boron does not have vacant d orbitals, a similar species containing boron is not known.
Reaction with acids and alkalis
Boron is unreactive towards acids and alkalis. Aluminium, however, dissolves in both acids and alkalis, liberating hydrogen.
⇒ \(2 \mathrm{Al}(\mathrm{s})+6 \mathrm{HCl}(\mathrm{aq}) \rightarrow 2 \mathrm{AlCl}_3(\mathrm{aq})+3 \mathrm{H}_2(\mathrm{~g})\)
⇒ \(2 \mathrm{Al}(\mathrm{s})+2 \mathrm{NaOH}(\mathrm{aq})+6 \mathrm{H}_2 \mathrm{O}(\mathrm{l}) \rightarrow \underset{\text { sodium tetrahydroxoaluminate (III) }}{2 \mathrm{Na}\left[\mathrm{Al}(\mathrm{OH})_4\right](\mathrm{aq})+3 \mathrm{H}_2(\mathrm{~g})}\)
Boron
Boron is a hard solid and exists in different allotropic forms. It is dark brown to black and has low electrical conductivity.
Anomalous behaviour
- As with Groups 1 and 2, the tilt member of Group 13 is different from the other members in certain aspects.
- The main reasons for the anomalous behaviour are the small size of the boron atom and the nonavailability of d orbitals.
- Boron, apart from the differences with Al( Ga, In and Tl, also exhibits a diagonal relationship with silicon.
Some of the main differences in the characteristics of boron from the rest of the members of the group are as follows.
- B is a nonmetal whereas Al, Ga, In and Tl are metals.
- B2O3 (like SiO2) is an acidic oxide, in contrast to Al2O3, which is amphoteric.
- Boric acid, H3BO3, which is represented as B(OH)3, is acidic whereas Al(OH)3 is amphoteric.
- Borates and silicates polymerise to form chains, rings and sheet structures. Aluminium forms no such compounds.
- The hydrides of B and Si are gaseous, readily hydrolysed and inflammable whereas aluminium hydride is a nonvolatile polymeric solid.
- BC13 is monomeric whereas the analogous aluminium compound (Al2Cl6) is dimeric in the solid state.
Chemical Properties
Boron has three outer electrons but it does not form B3+ ions (B is always covalent) due to its small size and high ionisation enthalpy. Pure crystalline boron is quite unreactive.
However, amorphous boron is more reactive. It reacts with dioxygen at a high temperature to form boron trioxide.
⇒ \(4 \mathrm{~B}+3 \mathrm{O}_2 \stackrel{973 \mathrm{~K}}{\longrightarrow} 2 \mathrm{~B}_2 \mathrm{O}_3\)
Boron combines with halogens at elevated temperatures to form the respective trihalides.
⇒ \(2 \mathrm{~B}+3 \mathrm{X}_2 \stackrel{\text { heat }}{\longrightarrow} 2 \mathrm{BX}_3\)
As already stated, the trihalides formed by Group 13 elements are electron-deficient compounds.
The bonded boron atom is surrounded by six electrons (three from B and one each from the three halogen atoms).
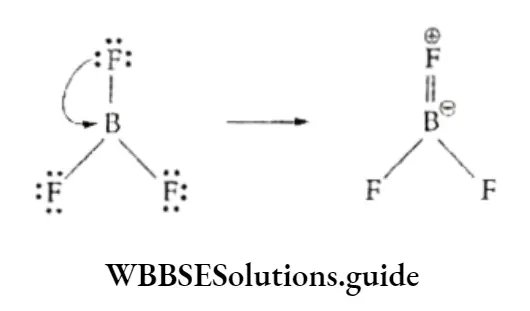
Boron halides act as Lewis acids and readily accept an electron pair from Lewis bases to complete the octet.
The boron trihalides, despite being electron deficient, are stable and do not dimerise.
They remedy the electron deficiency by forming a pπ-pπ bond, where tine empty 2p orbital on boron accepts nonbonding electrons of the halogen, as shown.
The molecule is a resonance hybrid of three canonical forms.
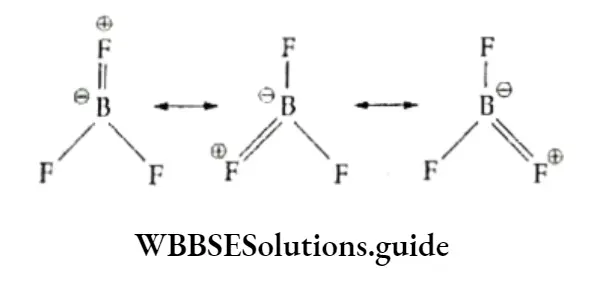
Thus the boron-fluorine bond length in BF3 is shorter than that of a single bond.
On heating to a very high temperature boron reacts with dinitrogen to form boron nitride, which is a slippery, white solid having a layered structure like that of graphite.
⇒ \(2 \mathrm{~B}+\mathrm{N}_2 \rightarrow 2 \mathrm{BN}\)
When fused with sodium hydroxide boron forms borates, and liberates hydrogen.
⇒ \(2 \mathrm{~B}+6 \mathrm{NaOH} \rightarrow 2 \mathrm{Na}_3 \mathrm{BO}_3+3 \mathrm{H}_2\)
Boron combines with many metals (not Group 1) to form borides, which are hard, high-melting solids.
⇒ \(\begin{aligned}
3 \mathrm{Mg}+2 \mathrm{~B} & \rightarrow \mathrm{Mg}_3 \mathrm{~B}_2 \\
\mathrm{Cr}+\mathrm{B} & \rightarrow \mathrm{CrB}
\end{aligned}\)
Boron does not react with nonoxidising acids like hydrochloric acid. However, it forms boric acid with hot concentrated oxidising acids.
⇒ \(\begin{aligned}
2 \mathrm{~B}+3 \mathrm{H}_2 \mathrm{SO}_4 & \rightarrow 2 \mathrm{H}_3 \mathrm{BO}_3+3 \mathrm{SO}_2 \\
\mathrm{~B}+3 \mathrm{HNO}_3 & \rightarrow \mathrm{H}_3 \mathrm{BO}_3+3 \mathrm{NO}_2
\end{aligned}\)
Boron acts as a reducing agent, reducing carbon dioxide and silica to carbon and silicon respectively.
Uses
- Boron is a hard, refractory solid having low density and low electrical conductivity.
- It is used to increase the hardness of steel, and crystalline boron is used in transistors.
- The isotope 10 B is a good neutron absorber and is used to make shields and control rods in nuclear reactors.
- Boron fibres are used in aircraft and for making bullet¬ proof vests. Borax, orthoboric acid and diboranes are some of the important compounds of boron which we will discuss here.
Borax
Borax, or sodium tetraborate decahydrate, is the most important compound of boron.
Initially formulated as Na 2B4O7-10H2O, it is now correctly represented as Na2[B405 (0H4)]-8H20. It naturally occurs in certain lakes in India, Tibet and the USA.
Preparation
Borax can be prepared from the mineral colemanite by boiling it with sodium carbonate solution.
⇒ \(\underset{\text { colemanite }}{\mathrm{Ca}_2 \mathrm{~B}_6 \mathrm{O}_{11}(\mathrm{~s})}+\underset{\mathrm{Na}_2 \mathrm{CO}_3(\mathrm{aq})}{2} \underset{\text { borax }}{\mathrm{Na}_2 \mathrm{~B}_4 \mathrm{O}_7(\mathrm{aq})}+\underset{\text { sodium metaborate }}{2 \mathrm{NaBO}_2(\mathrm{aq})}+2 \mathrm{CaCO}_3(\mathrm{~s})\)
Calcium Carbonate Is Filtered Off. The Filtrate, On Concentration, Gives Crystals Of Borax. Carbon Dioxide Is Passed Through The Mother Liquor To Convert Sodium Metaborate To Borax.
⇒ \(4 \mathrm{NaBO}_2+\mathrm{CO}_2 \rightarrow \mathrm{Na}_2 \mathrm{~B}_4 \mathrm{O}_7+\mathrm{Na}_2 \mathrm{CO}_3\)
Properties and reactions
Borax is a white, crystalline solid slightly soluble in cold water but more soluble in hot water. It hydrolyses to give an alkaline solution
⇒ \(
\mathrm{Na}_2 \mathrm{~B}_4 \mathrm{O}_7+7 \mathrm{H}_2 \mathrm{O} \rightleftharpoons 2 \mathrm{NaOH}+4 \mathrm{H}_3 \mathrm{BO}_3
orthoboric acid\)
“What are p-Block elements, their properties, and uses?”
On heating, borax loses its water of crystallisation and swells to give a white, opaque, puffy mass of the anhydrous salt, which on further heating gives a glassy mass comprising sodium metaborate and boron trioxide.
⇒ \(\mathrm{Na}_2 \mathrm{~B}_4 \mathrm{O}_7 \cdot 10 \mathrm{H}_2 \mathrm{O} \stackrel{-10 \mathrm{H}_2 \mathrm{O}}{\longrightarrow} \mathrm{Na}_2 \mathrm{~B}_4 \mathrm{O}_7 \stackrel{\text { heat }}{\longrightarrow} 2 \mathrm{NaBO}_2+\mathrm{B}_2 \mathrm{O}_3\)
Boron trioxide is acidic and reacts with metallic (basic) oxides, forming salts called metaborates.
The metaborates have characteristic colours and this reaction is the basis of the borax bead test for the detection of metal ions.
⇒ \(\mathrm{MO}+\mathrm{B}_2 \mathrm{O}_3 \stackrel{\text { heat }}{\longrightarrow} \mathrm{M}\left(\mathrm{BO}_2\right)_2\)
The test is performed on a metal salt by heating it with borax in the loop of a platinum wire.
On heating, the metal salt forms an oxide. This oxide fuses with the boron trioxide obtained from borax to form a metaborate.
When borax is treated with a calculated quantity of sodium hydroxide, sodium metaborate is formed.
⇒ \(\mathrm{Na}_2 \mathrm{~B}_4 \mathrm{O}_7+2 \mathrm{NaOH} \rightarrow 4 \mathrm{NaBO}_2+\mathrm{H}_2 \mathrm{O}\)
Borax, on treatment with a calculated quantity of concentrated sulphuric acid, gives boric acid.
⇒ \(\begin{array}{r}
\mathrm{Na}_2 \mathrm{~B}_4 \mathrm{O}_7+\mathrm{H}_2 \mathrm{SO}_4 \rightarrow \mathrm{Na}_2 \mathrm{SO}_4+\mathrm{H}_2 \mathrm{~B}_4 \mathrm{O}_7 \\
\downarrow \\
\downarrow \mathrm{H}_2 \mathrm{O} \\
4 \mathrm{H}_3 \mathrm{BO}_3
\end{array}\)
Uses
Borax is used
- In the manufacture of enamels and glazes for the manufacture of tiles
and in pottery, - In making optical glass and heat-resistant glass (borosil, pyrex, etc.),
- In medicinal soaps and eye washes due to its antiseptic properties, and
- In the borax bead test for the detection of metals.
Structure
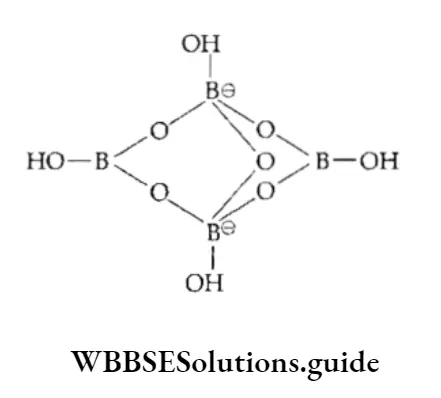
Borax (Na2[B405(0H)4]-8H20) is made up of two triangular units and two tetrahedral units. The ion is actually [B405(0H)4]2_. The other water molecules are associated with the metal ions.
Boric acids
Orthoboric acid, H3 B03, commonly known as boric acid, and metaboric acid, HBO 2, are two common oxoacids of boron.
Orthoboric acid occurs naturally in the condensate from volcanic steam vents called stuffing.
Preparation
Boric acid can be prepared by acidifying an aqueous solution of borax with sulphuric acid or hydrochloric add.
On cooling, the crystals of boric add separate out.
⇒ \(\begin{gathered}
\mathrm{Na}_2 \mathrm{~B}_4 \mathrm{O}_7+\mathrm{H}_2 \mathrm{SO}_4+5 \mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{Na}_2 \mathrm{SO}_4+4 \mathrm{H}_3 \mathrm{BO}_3 \\
\mathrm{Na}_2 \mathrm{~B}_4 \mathrm{O}_7+2 \mathrm{HCl}+5 \mathrm{H}_2 \mathrm{O} \rightarrow 2 \mathrm{NaCl}+4 \mathrm{H}_3 \mathrm{BO}_3
\end{gathered}\)
It can also be obtained when sulphur dioxide is passed through a hot solution of colemanite in water. The resulting solution, on cooling, gives crystals of boric acid.
⇒ \(\mathrm{Ca}_2 \mathrm{~B}_6 \mathrm{O}_{11}+4 \mathrm{SO}_2+11 \mathrm{H}_2 \mathrm{O} \rightarrow 2 \mathrm{Ca}\left(\mathrm{HSO}_3\right)_2+6 \mathrm{H}_3 \mathrm{BO}_3\)
The hydrolysis of some compounds of boron (viz., halide, nitride, hydride) gives boric acid.
⇒ \(\begin{aligned}
\mathrm{BCl}_3+3 \mathrm{H}_2 \mathrm{O} & \rightarrow \mathrm{H}_3 \mathrm{BO}_3+3 \mathrm{HCl} \\
\mathrm{BN}+3 \mathrm{H}_2 \mathrm{O} & \rightarrow \mathrm{H}_3 \mathrm{BO}_3+\mathrm{NH}_3 \\
\mathrm{~B}_2 \mathrm{H}_6+6 \mathrm{H}_2 \mathrm{O} & \rightarrow 2 \mathrm{H}_3 \mathrm{BO}_3+6 \mathrm{H}_2
\end{aligned}\)
Properties And Reactions
Orthoboric acid is a flaky, white, crystalline solid, moderately soluble in water.
It is a very weak acid. Unlike acids, which donate protons, they accept a pair of electrons from OH ions and thus behave as a Lewis acid.
⇒ \(\mathrm{H}_3 \mathrm{BO}_3+2 \mathrm{H}_2 \mathrm{O} \rightleftharpoons \underset{\text { metaborate ion }}{\left[\mathrm{B}(\mathrm{OH})_4\right]^{-}}+\mathrm{H}_3 \mathrm{O}^{+}\)
On heating orthoboric acid loses water to form metaboric add, which on further heating yields tetraboric acid and finally boron trioxide.
⇒ \(\underset{\text { orthoboric acid }}{\mathrm{H}_3 \mathrm{BO}_3} \frac{-\mathrm{H}_2 \mathrm{O}}{375 \mathrm{~K}} \underset{\text { metaboric acid }}{\mathrm{HBO}_2}\)
Uses
Boric acid is used
- As an antiseptic as well as a preservative, and
- In the manufacture of heat-resistant glass, enamels and glazes in pottery.
Structure
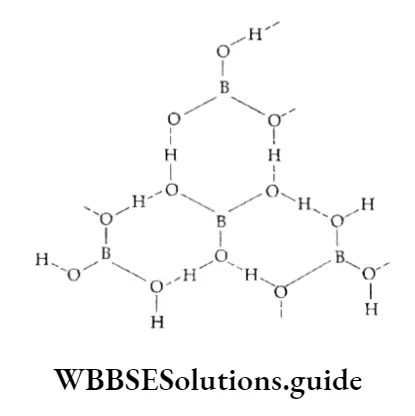
Orthoboric add contains triangular B033” units, which are hydrogen bonded to give a layered structure.
The layers are quite a distance apart (318 pm) and the crystal breaks easily, thus making the fr’ compound soft and flaky.
Boron hydrides
Boron forms a series of volatile hydrides collectively called boranes. Given its trivalency, boron is expected to form a simple hydride BH3.
However, no such compound is known, the simplest hydride is diborane (B2H6). Two series of boranes are known corresponding to the stoichiometries—BnHn+4 and BnHn+6.
The most important hydride is diborane. Its preparation, properties and structure are discussed as follows.
Preparation
1. Diborane can be prepared by the reduction of boron trifluoride etherate (Et20-BF3) with lithium aluminium hydride (LiAlH4) in ether or with sodium borohydride (NaBH4) in diglyme (CH3OCH2CH2OCH2CH2OCH3) as a solvent.
⇒ \(4 \mathrm{Et}_2 \mathrm{O} \cdot \mathrm{BF}_3+3 \mathrm{LiAlH}_4 \stackrel{\text { ether }}{\longrightarrow} 2 \mathrm{~B}_2 \mathrm{H}_6+3 \mathrm{LiF}+3 \mathrm{AlF}_3+4 \mathrm{Et}_2 \mathrm{O}\)
⇒ \(4 \mathrm{Et}_2 \mathrm{O} \cdot \mathrm{BF}_3+3 \mathrm{NaBH}_4 \stackrel{\text { diglyme }}{\longrightarrow} 2 \mathrm{~B}_2 \mathrm{H}_6+3 \mathrm{Na}\left[\mathrm{BF}_4\right]+4 \mathrm{Et}_2 \mathrm{O}\)
In the laboratory, diborane is prepared by heating sodium borohydride and iodine in the solvent diglyme.
⇒ \(2 \mathrm{NaBH}_4+\mathrm{I}_2 \stackrel{\text { diglyme }}{\longrightarrow} \mathrm{B}_2 \mathrm{H}_6+2 \mathrm{NaI}+\mathrm{H}_2\)
Diborane is industrially prepared by the reduction of boron trifluoride with sodium hydride.
⇒ \(2 \mathrm{BF}_3+6 \mathrm{NaH} \stackrel{450 \mathrm{~K}}{\longrightarrow} \mathrm{B}_2 \mathrm{H}_6+6 \mathrm{NaF}\)
Properties and reactions
Diborane is a colourless, highly reactive gas. It catches fire spontaneously in the air and explodes in oxygen. The reaction is highly exothermic.
⇒ \(\mathrm{B}_2 \mathrm{H}_6+3 \mathrm{O}_2 \rightarrow \mathrm{B}_2 \mathrm{O}_3+3 \mathrm{H}_2 \mathrm{O} \quad \Delta_c H^{\ominus}=-2165 \mathrm{~kJ} \mathrm{~mol}^{-1}\)
It has a higher heat of combustion per unit weight than most other fuels and was earlier used as a rocket fuel.
However, this was discontinued as the combustion was incomplete and the exhaust nozzles became blocked with I, a nonvolatile polymer.
Diborane is rapidly hydrolysed to yield boric acid and hydrogen.
⇒ \(\mathrm{B}_2 \mathrm{H}_6+6 \mathrm{H}_2 \mathrm{O} \rightarrow 2 \mathrm{H}_3 \mathrm{BO}_3+6 \mathrm{H}_2\)
When heated in a sealed tube a complex reaction occurs and the borane is converted to various higher boranes, depending on the prevailing experimental conditions.
⇒ \(\begin{aligned}
& \mathrm{B}_2 \mathrm{H}_6 \stackrel{373-523 \mathrm{~K}}{\longrightarrow} \mathrm{B}_4 \mathrm{H}_{10}+\mathrm{B}_5 \mathrm{H}_{11}+\mathrm{B}_6 \mathrm{H}_{12}+\mathrm{B}_{10} \mathrm{H}_{14} \\
& \mathrm{~B}_2 \mathrm{H}_6 \stackrel{353-523 \mathrm{~K}}{\underset{\text { high pressure }}{\longrightarrow}} \mathrm{B}_4 \mathrm{H}_{10} \\
& \mathrm{~B}_2 \mathrm{H}_6 \stackrel{423 \mathrm{~K}}{\longrightarrow} \mathrm{B}_{10} \mathrm{H}_{14}
\end{aligned}\)
“p-Block elements, characteristics, applications, and examples”
When diborane, which is a Lewis acid, is treated with Lewis bases, like amines [(CH3)2NH or (CH3)3N], it undergoes cleavage to give BH3, which then forms an adduct with the amine. (Here Me represents the methyl I group.
⇒ \(\mathrm{B}_2 \mathrm{H}_6+2 \mathrm{NMe}_3 \rightarrow 2 \mathrm{BH}_3 \cdot \mathrm{NMe}_3\)
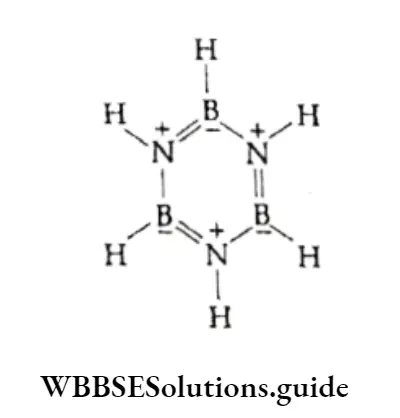
⇒ \(\mathrm{B}_2 \mathrm{H}_6+\mathrm{NH}_3 \longrightarrow \underset{\substack{\mathrm{B}_3 \mathrm{~N}_3 \mathrm{H}_6+\mathrm{H}_2 \\ \text { borazine }}}{\left[\mathrm{BH}_2\left(\mathrm{NH}_3\right)_2\right]^{+}\left[\mathrm{BH}_4\right]}\)
On the reaction of diborane with ammonia, initially, an addition V product B2H6 2NH3 (better formulated as [BH2(NH3)21+[(BH4))-) is formed, which decomposes on heating to form a volatile compound, borazine. Borazine is isoelectronic and isostructural with benzene.
Diborane reacts with alkali metal hydrides to form tetrahydroborates or borohydrides, which contain the tetrahedral BH4-
⇒ latex]2 \mathrm{NaH}+\mathrm{B}_2 \mathrm{H}_6 \underset{\text { ether }}{\stackrel{\text { diethyl }}{\longrightarrow}} 2 \mathrm{NaBH}_4[/latex]
Lithium borohydride and sodium borohydride are reducing agents used in organic synthesis.
Diborane undergoes addition reactions with alkenes and alkynes in ether at room temperature to form organoboranes.
⇒ \(6 \mathrm{RCH}=\mathrm{CH}_2+\mathrm{B}_2 \mathrm{H}_6 \longrightarrow 2 \mathrm{~B}\left(\mathrm{CH}_2 \mathrm{CH}_2 \mathrm{R}\right)_3\)
This is referred to as a hydroboration reaction. Organoboranes are useful starting materials for the synthesis of various organic compounds.
Structure
The structure of diborane is quite different from the one usually expected, as it cannot be explained on the basis of simple theories of bonding.
The electronic configuration of boron ([He]2s2 2p1) suggests that there are three valence electrons which implies that a boron atom can form three covalent bonds.
But, if each boron atom is bonded to three hydrogen atoms, then there will be no electron left to hold the two BH3 units together. The structure of diborane has been confirmed by electron diffraction studies.
Diborane is an electron-deficient compound as the total number of valence electrons (12) is not sufficient to form eight bonds.
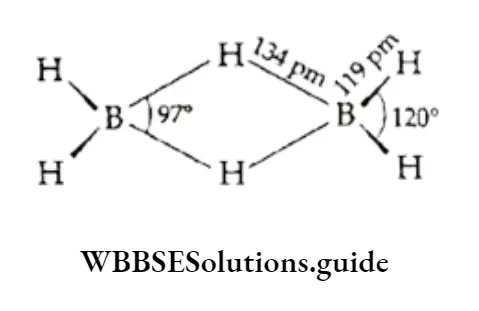
The two hydrogen atoms which hold the boron atoms together are called the bridging hydrogen atoms whereas the other four hydrogen atoms are called the terminal hydrogen atoms.
The bridging hydrogen atoms, being in the plane perpendicular to that of the rest of the atoms, prevent rotation between the two boron atoms. The terminal B —H distance is the same as in a non-electron-deficient compound (119 pm).
The terminal B—H bonds are formed by sharing an electron pair between boron and hydrogen and are referred to as two-centre two-electron (2c-2e) bonds. The bridge bonds, B—H—B, are different from the normal covalent bonds.
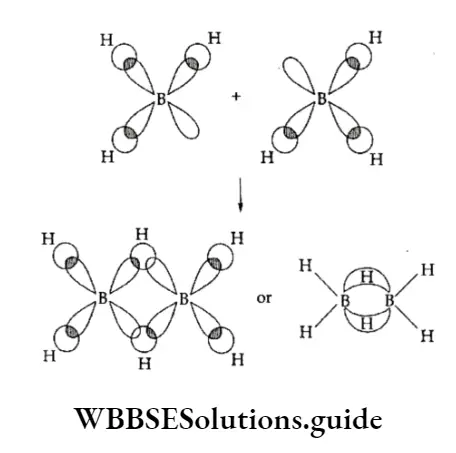
Since the four terminal B —H bonds use eight out of twelve valence electrons, only four valence electrons are present to form the four bridge bonds. The formation of the bridge bond can be understood from the concept of hybridisation.
Each B atom which is sp3 hybridised contains four hybrid orbitals, one of which is vacant and the others singly filled by the three valence electrons.
Two of these orbitals of each atom overlap with the Is orbital of hydrogen to form the terminal B—H bonds.
One singly filled sp3 hybrid orbital on one boron atom and a vacant sp3 hybrid orbital on another boron atom overlap with the singly filled Is orbital of hydrogen to form a bonding orbital, B —H—B, shaped like a banana, covering all the three atoms.
Thus two electrons hold three atoms together and this is called a three-centre two-electron bond (3c-2e)
Aluminium
Aluminium is a moderately soft, light, nontoxic, corrosion-resistant metal. It is the most abundant metal. It has high thermal and electrical conductivity and tensile strength. Many of its mechanical properties are improved by alloying it with copper, manganese, magnesium, silicon and zinc.
Aluminium and its alloys are used in making utensils and as a structural material for aircraft, ships and the transportation industry. The metal is also used for making electric cables and in the packaging industry mainly as metal-foil cans and toothpaste tubes.
Aluminium is used as a reducing agent in the aluminothermic process for the extraction of chromium and manganese from their ores.
Alums are double salts comprising aluminium sulphate and an alkali metal sulphate. They may be A represented by the general formula: M2S04′ A12(S04)3-24H20 or 2MA1(S04)2-12H20 (M = Na or K).
Alums are in water purification and as a mordant in the dyeing and printing of textiles. (Mordants are inorganic oxides salts, which are absorbed on the fabric.)
Aluminium oxide exists in various crystalline modifications, some of which are used as the adsorbent in thin-layer chromatography and as catalyst support. Some others like corundum are used as refractory materials and abrasives.
Group 14 Elements
The elements in this group are carbon, silicon, germanium, tin and lead. A marked increase in metallic character is noted on descending the group. The first two elements are nonmetals while the others are metals.
Occurrence
Carbon is the seventeenth-most abundant element in the earth’s crust. In the free state, it is present as coal, diamond and graphite, while in the combined state it is present as carbon dioxide, carbonate rocks and hydrocarbons (petroleum, natural gas).
Carbon is an essential constituent of all living systems. Naturally occurring carbon has two stable isotopes—12C and 13C. In addition, a radioactive isotope, 14 C, of half-life 5770 years is also found.
Silicon is the second-most abundant element in the earth’s crust, next only to oxygen. It is present as silica (sand) and silicates (rocks). Germanium is a rare element occurring in traces in coal, lead and zinc ores. Tin occurs mainly as cassiterite (SnO2) and lead as galena (PbS).
Electronic configuration
The valence-shell electronic configuration of Group 14 elements is n2np2.
The inner core of carbon and silicon contains only s and p electrons whereas that of germanium, tin and lead contains d electrons also.
In lead, fourteen f electrons are also present. The presence of d and f electrons in some members of the group results in the variation of properties among the Group 14 elements.
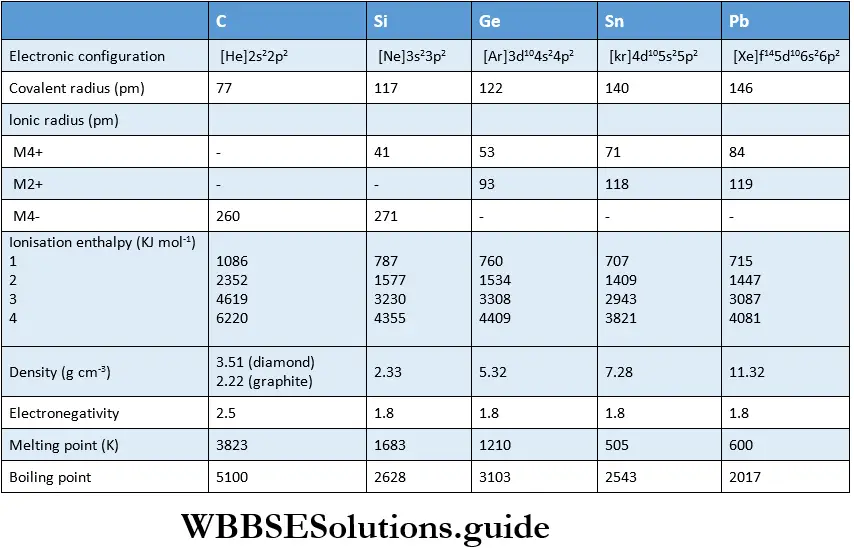
Covalent radii
The covalent radii increase down the group. There is a significant increase in covalent radius from carbon to silicon.
The covalent radius of germanium is only slightly more than that of silicon. This is due to the presence of ten d electrons in germanium, which do not shield the nuclear charge effectively.
A small increase in radius is noted on descending the group, i.e., between tin and lead. This is due to the presence of d and f electrons in the inner core. If you recall, such a behaviour was noted in Group 13 also.
Ionisation enthalpy
The ionisation enthalpies of the Group 14 elements are higher than those of the corresponding Group 13 elements.
This is expected as ionisation enthalpy increases across a period due to an increase in nuclear charge and a decrease in the size of the element, making the electrons tightly held.
The variation in ionisation enthalpy is not very regular though a decrease is noted in the first and second ionisation enthalpies on moving from carbon to tin. The irregularities occur due to the poor shielding effect of d and f orbitals.
Electronegativity
The elements of this group are more electronegative than the corresponding members of Group 13 due to their smaller size.
The electronegativity of carbon is more than that of the other members of the group.
Owing to this it can gain electrons to form anions like C2~ (carbide), C22 (acetylide) and C4~ (methanide). The electronegativity values of the other elements are almost the same.
Oxidation states
The common oxidation states are +4 and +2, the latter arising due to the inert-pair effect, which is common in the heavier members of the group. The sum of the first four ionisation enthalpies is immense.
Therefore, the elements in the +4 oxidation state generally form covalent compounds. On descending the group, the +4 oxidation state becomes less stable, and the +2 oxidation state is shown increasingly.
This is also reflected in the oxidising power of the tetravalent compounds of the heavier elements of the group.
If we consider the oxides CO2, SiO2and GeO2, they are not oxidising; SnO2 is a mild oxidising agent while PbO2 is a strong oxidising agent.
The tendency to form ionic compounds in the +2 oxidation state increases on moving down the group.
PbF2 and PbCl 2 are ionic compounds. To sum up, both carbon and silicon exhibit mostly the +4 oxidation state; the +4 state is more stable for germanium than the +2 state.
Tin shows both the oxidation states whereas the +2 state is stable for lead.
Chemical Reactivity
The elements of Group 14 are, in general, relatively unreactive. Among the members of the group, the heavier ones are more reactive than the lighter ones.
Reaction With Air
All the elements of the group form oxides when heated with oxygen. Generally, monoxides (MO) and dioxides (MOz) are formed.
⇒ \(\begin{gathered}
2 \mathrm{M}+\mathrm{O}_2 \stackrel{\text { heat }}{\longrightarrow} 2 \mathrm{MO} \\
\mathrm{M}+\mathrm{O}_2 \stackrel{\text { heat }}{\longrightarrow} \mathrm{MO}_2
\end{gathered}\)
The dioxides are all thermally stable except PbO2. This is because of the inert-pair effect, which enhances the stability of the +2 oxidation state in lead. PbO2 is thus a good oxidising agent.
The acidic nature of dioxides decreases down the group as the nonmetallic character of the elements decreases—CO2, SiO2 and GeO2 are acidic whereas SnO2 and PbO2 are amphoteric.
CO2 is stable. SiO2 however exists only at a high temperature. The monoxides of Ge, Sn and lead are known. GeO is reducing in nature.
Reaction with water
Most of the Group 14 elements are unaffected by water. Tin reacts with steam.
⇒ \(\mathrm{Sn}+2 \mathrm{H}_2 \mathrm{O} \stackrel{\text { heat }}{\longrightarrow} \mathrm{SnO}_2+2 \mathrm{H}_2\)
Lead does not undergo this reaction as it gets superficially oxidised and a protective oxide film is formed on the surface.
Reaction with halogens
The elements in this group form tetrahalides (MX2) as well as dihalides (MX2) (X = F, C1, Br, I), All tetrahalides are known except Pbl 4.
The bond enthalpy of Pb—I is not enough to compensate for the energy required to unpair the 6s2 electrons of lead and get four impaired electrons.
The stability of the tetrahalides decreases down the group and that of dihalides increases (inert-pair effect).
The fluorides, by the high electronegativity of fluorine, have appreciable ionic character.
SnF4 and PbF4 exist as ionic solids. The other tetrahalides are covalent. The central metal atom in tetrahalides is sp3 hybridised and the shape of the molecule is tetrahedral.
The Group 14 elements other than carbon can expand their covalence from four due to the presence of d orbitals.
Since complex formation by elements is favoured by the availability of empty orbitals, carbon does not form complexes. A complex is a compound in which molecules or ions form coordinate bonds with a metal atom or ion.
Tetrahalides of carbon are inert towards water (hydrolysis takes place by complex formation), but tetrahalides of silicon undergo hydrolysis readily.
⇒ \(\mathrm{SiCl}_4+4 \mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{Si}(\mathrm{OH})_4+4 \mathrm{HCl} sillicic acid\)
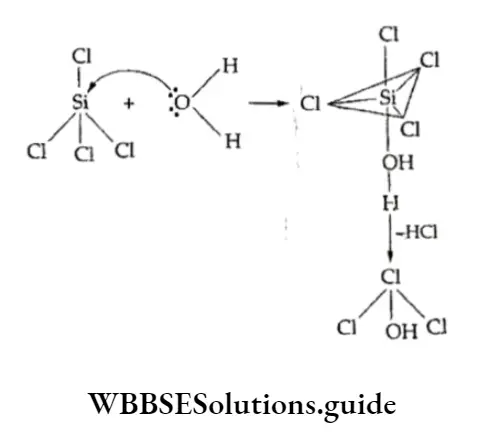
The hydrolysis proceeds via the formation of an intermediate, in which a water molecule gets attached to the Si atom before a Si— —CI bond is broken.
Thus, the Si atom shows a coordination number of five and the lone pair of electrons present on the oxygen of the water molecule is accommodated in a vacant 3d orbital of the silicon atom.
(The coordination number is the number of species surrounding a given atom or ion in a complex.)
The process continues till all Si—Cl bonds are replaced by Si—OH bonds. The other trihalides of the group also tend to hydrolyse but the hydrolysis can be suppressed by adding the appropriate halogen acid.
The tetrahalides are all examples of electron-precise molecules, i.e., the central metal is surrounded by an octet of electrons (4 from the Group 14 element and 1 each from the four halogens).
They are neither electron-deficient nor electron-rich species and thus do not behave as Lewis acids or bases. Carbon cannot extend its covalence beyond four due to the absence of d orbitals.
The tetrahalides of Si, Ge, Sn and Ph can increase their coordination number to 6 by forming complexes like [SiF6]-2 and [SnCl-6]2-.
In these species, the central atom accepts electron pairs from donors and is spM1 hybridised. Germanium, tin and lend form dihalides. There is an increase in the stability of dihalides on moving down the tyre group.
Carbon
Anomalous behaviour
Like in other groups, carbon, being the first member of Group 14, differs from the other members in many aspects.
The main reasons for these differences are
- The small size of the carbon atom,
- High electronegativity,
- Unavailability of d orbitals and
- High ionisation enthalpy.
Some of the specific anomalies are that carbon has a maximum covalence of four and is the only element to form stable pπ-pπ bonds.
This apart, carbon has a remarkable property of forming long chains and rings of its atoms.
This is called catenation and is attributed to the strength of the C—C bond. The tendency to catenate decreases down the group with the decrease in the strength of the M —M bond.
The bond enthalpy decreases with the decrease in electronegativity and increase in the atomic size of elements.
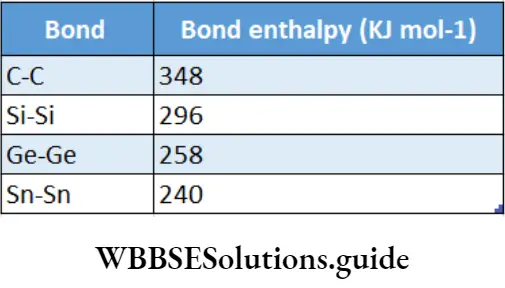
Carbon forms pz-pir multiple bonds with itself and other small electronegative elements, e.g., C=C, C=C, C=0, C=S and C=N.
The pn-pz bonds are formed by the sideways overlap of a p orbital of carbon with a p orbital of another carbon, oxygen or nitrogen atom.
The heavier elements do not form pπ-pπ bonds as the orbitals are too large and diffuse to allow effective overlap. However, they can form p5-d5 bonds.
Allotropes
Due to its unique property of catenation and pn-pz bond formation, carbon exists in different allotropic modifications.
Until 1985, only two crystalline allotropes of carbon were known—diamond and graphite. In 1985, another allotrope of carbon known as fullerene was discovered by HW Krato, E Smalley and RF Curl.
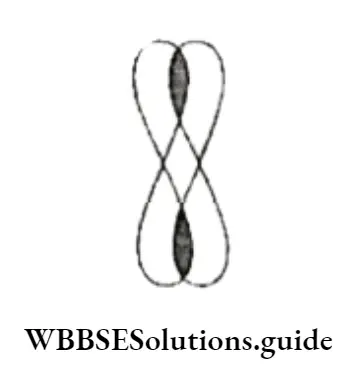
Diamond
Diamond is the hardest substance known and has a very rigid structure. Each carbon atom in the crystalline lattice is sp3 hybridised and linked covalently to the tour other carbon atoms. Tire C—C distance is 154 pm.
The rigid three-dimensional structure extends throughout the lattice, which is difficult to break, making diamond t extremely hard.
Graphite
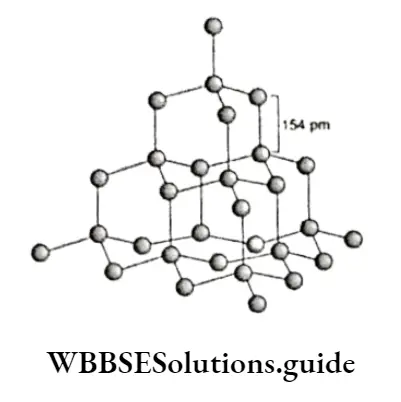
Unlike diamond, graphite has a layered structure.
Individual layers of the fused hexagonal rings of carbon atoms are held together by weak van der Waals forces.
Each carbon atom shared by three hexagons is sp2 hybridised. Out of the four valence electrons, the toe is involved in bond formation and the fourth electron is involved in delocalised re-bonding.
The C—C bond length within a layer is 141.5 pm while the inter-layer distance is 335.4 pm.
Due to weak van der Waals forces the layers can slide over one another and the jt electrons move within each layer, making graphite a good conductor of electricity and conferring lubricating properties.
Graphite cleaves readily between the layers and is soft and slippery. Some physical properties of diamond and graphite axes are summarised.
Fullerenes
A fascinating discovery was the synthesis of spheroidal carbon-cage molecules called fullerenes.
The discoverers of fullerene were awarded the Nobel Prize in Chemistry (1996). Fullerenes were first prepared by the evaporation of graphite using a laser.
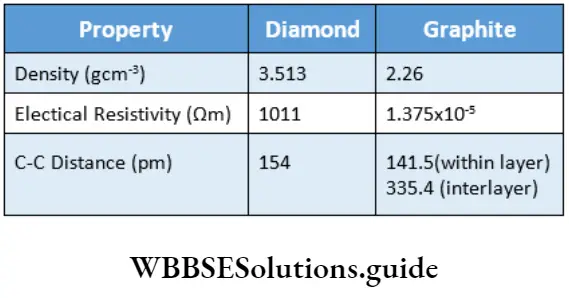
A more practical method for the preparation is to heat graphite in an electric arc in an inert atmosphere (helium or argon).
A sooty material so formed consists of C with small amounts of C70 and other fullerenes containing an even number of carbon atoms upto 350.
Fullerenes have a smooth structure and, unlike diamond and graphite, dissolve in organic solvents like toluene.
C60 is the most stable fullerene. It has the shape of a football and is called buckminsterfullerene.
“Uses of p-Block elements, their properties, and significance”
consists of fused five- and six-membered carbon rings. Each six-membered ring is surrounded alternately by hexagons and pentagons of carbon while the five-membered rings are fused to five hexagons.
The carbon atoms are sp2 hybridised, each carbon atom forms three bonds with the other three carbon atoms and the fourth electron is delocalised. Although all atoms are equivalent, the bonds are not.
In the structure, C —C bonds of two different bond lengths occur—at the fusion of two six-membered rings (C—C bond length 135.5 pm) and at the fusion of five- and six-membered rings (C—C bond length 146.7 pm).
The smallest known fullerene is C2O, which is obtained from the hydrocarbon C2OH2O by a two-step reaction. First, the hydrogens are replaced by bromine. This is then followed by denomination.
Thermodynamically, the most stable allotrope of carbon is considered to be graphite. It is considered to be the standard state for carbon and its enthalpy of formation is taken as zero.
The enthalpies of the formation of diamond and fullerene are 1.90 and 38.1 kJ mol-1 respectively. In addition to these forms, there are a few other elemental forms of carbon like coke, lampblack, soot and charcoal. These are impure forms of graphite.
Uses
- The isotope 12C has been assigned a mass of 12.0000 units and is the international standard for atomic mass measurements.
- The isotope 14C is radioactive (half-life 5770 years). It is, therefore, used in radiocarbon dating, a method used to determine the age of samples of organic origin.
- Diamond is used as a gemstone. Inferior quality diamonds are used as abrasives in cutting, drilling, and V also in sharpening and polishing tools. Diamond is also used for making dies for drawing thin wires from metals.
- Graphite conducts electricity. It is, therefore, used for making electrodes in batteries and electrolytic cells. It is used in steel making, for making acid- and alkali-resistant crucibles and as a moderator in atomic reactors. It is also used in the lead of pencils and as a lubricant (being soft and slippery). Graphite fibres embedded in, plastic are used in tennis rackets, aircraft and fishing rods.
- Activated charcoal is an excellent adsorbent. It is, therefore, used in gas masks (to adsorb toxic gases) and in air-conditioning systems to control odour. It is also used in the manufacture of sugar, refining of oils and in water filters. (When charcoal is activated for adsorption by steaming or heating in a vacuum, it is known as activated charcoal.)
- Coke is a fuel and also finds use as a reducing agent in metallurgy.
- Carbon black is used as a pigment in black ink and as a filler in tyres.
Oxides Of Carbon And Silicon
Among all compounds of carbon and silicon, oxides are the most important and abundant.
The oxides of carbon-carbon monoxide and carbon dioxide differ from the others as they contain pn-pn multiple bonds between carbon and oxygen.
Silicon forms two oxides—silicon monoxide and silicon dioxide, also called silica.
Carbon monoxide
It is obtained when carbon is burnt in a limited supply of air.
⇒ \(2 \mathrm{C}(\mathrm{s})+\mathrm{O}_2(\mathrm{~g}) \stackrel{\text { heat }}{\longrightarrow} 2 \mathrm{CO}(\mathrm{g})\)
It is also obtained during the incomplete combustion of fuels like petrol and diesel and is thus always present in automobile exhausts. Pure carbon monoxide is obtained by the dehydration of formic acid with concentrated sulphuric acid at 373 K.
⇒ \(\mathrm{HCOOH} \stackrel{\text { heat }}{\stackrel{\text { canc. } \mathrm{H}_2 \mathrm{SO}_4}{\longrightarrow}} \mathrm{CO}+\mathrm{H}_2 \mathrm{O}\)
It is commercially obtained by passing steam over red hot coke. The reaction also produces hydrogen along with carbon monoxide, and this mixture is called water gas or synthetic gas. Water gas is used as a fuel.
⇒ \(\mathrm{C}(\mathrm{s})+\mathrm{H}_2 \mathrm{O}(\mathrm{g}) \stackrel{473-1273 \mathrm{~K}}{\longrightarrow} \underbrace{\mathrm{CO}(\mathrm{g})+\mathrm{H}_2(\mathrm{~g})}_{\text {water gas }}\)
If air is listed instead of steam, a mixture of carbon monoxide and nitrogen is produced. This is called producer I gas and is an important fuel.
⇒ \(2 \mathrm{C}(\mathrm{s})+\underbrace{\mathrm{O}_2(\mathrm{~g})+4 \mathrm{~N}_2(\mathrm{~g})}_{\text {air }} \longrightarrow \underbrace{2 \mathrm{CO}(\mathrm{g})+4 \mathrm{~N}_2(\mathrm{~g})}_{\text {producer gas }}\)
Properties and reactions Of Carbon monoxide
Carbon monoxide is a colourless and odourless gas. It is a neutral oxide insoluble in water.
On combustion, carbon monoxide gives carbon dioxide. The reaction is highly exothermic and this explains why producer gas and water gas, which contain carbon monoxide, are important fuels.
Among the two, water gas is more efficient than producer gas because both carbon monoxide and hydrogen bum and evolve heat.
⇒ \(2 \mathrm{CO}(\mathrm{g})+\mathrm{O}_2(\mathrm{~g}) \rightarrow 2 \mathrm{CO}_2(\mathrm{~g}) \quad \Delta_c H^{\ominus}=-566 \mathrm{~kJ} \mathrm{~mol}^{-1}\)
Due to its strong affinity for oxygen, carbon monoxide is a powerful reducing agent and is used to extract many metals (except alkali or alkaline earth metals) by the reduction of their oxides.
⇒ \(\begin{gathered}
\mathrm{ZnO}(\mathrm{s})+\mathrm{CO}(\mathrm{g}) \rightarrow \mathrm{Zn}(\mathrm{s})+\mathrm{CO}_2(\mathrm{~g}) \\
\mathrm{Fe}_2 \mathrm{O}_3(\mathrm{~s})+3 \mathrm{CO}(\mathrm{g}) \rightarrow 2 \mathrm{Fe}(\mathrm{s})+3 \mathrm{CO}_2(\mathrm{~g}) \\
\mathrm{CuO}(\mathrm{s})+\mathrm{CO}(\mathrm{g}) \rightarrow \mathrm{Cu}(\mathrm{s})+\mathrm{CO}_2(\mathrm{~g})
\end{gathered}\)
Carbon monoxide contains a triple bond between the carbon atom and the oxygen atom—one CT and two n bonds.
The lone pair of electrons on carbon (C=O) may be donated to a transition metal in its low oxidation state to form a compound called metal carbonyl. You will learn more about these in higher classes.
⇒ \(
\mathrm{Ni}+4 \mathrm{CO} \rightarrow \mathrm{Ni}(\mathrm{CO})_4
nickel carbonyl\)
Carbon monoxide is very toxic. The toxicity is due to its ability to form a very stable complex (carboxyhaemoglobin) with the haemoglobin of blood.
Haemoglobin acts as an oxygen carrier as it binds itself to oxygen in the lungs and transports it.
it to the tissues. The formation of carboxyhaemoglobin prevents haemoglobin from binding itself to oxygen.
If a person is exposed to large amounts of carbon monoxide, he or she may eventually die (due to lack of oxygen).
Carbon dioxide
Preparation Of Carbon dioxide
It is obtained when carbon or fossil fuels are burnt in an excess of air. In other words, carbon dioxide is produced by the complete combustion of carbon and carbon-containing fuels.
⇒ \(\begin{gathered}
2 \mathrm{C}(\mathrm{s})+\mathrm{O}_2(\mathrm{~g}) \stackrel{\text { heat }}{\longrightarrow} \mathrm{CO}_2(\mathrm{~g}) \\
\mathrm{CH}_4(\mathrm{~g})+2 \mathrm{O}_2(\mathrm{~g}) \stackrel{\text { heat }}{\longrightarrow} 2 \mathrm{CO}_2(\mathrm{~g})+2 \mathrm{H}_2 \mathrm{O}(\mathrm{g}) \\
\mathrm{C}_5 \mathrm{H}_{12}(\mathrm{~g})+8 \mathrm{O}_2(\mathrm{~g}) \stackrel{\text { heat }}{\longrightarrow} 5 \mathrm{CO}_2(\mathrm{~g})+6 \mathrm{H}_2 \mathrm{O}(\mathrm{g})
\end{gathered}\)
Carbon dioxide can be prepared in the laboratory by the action of a dilute add-on carbonate. Generally, calcium carbonate in the form of marble chips is used.
⇒ \(\mathrm{CaCO}_3(\mathrm{~s})+2 \mathrm{HCl}(\mathrm{aq}) \longrightarrow \mathrm{CaCl}_2(\mathrm{aq})+\mathrm{H}_2 \mathrm{O}(\mathrm{l})+\mathrm{CO}_2(\mathrm{~g})\)
The large-scale production of carbon dioxide involves the thermal decomposition of limestone in lime kilns.
⇒ \(\mathrm{CaCO}_3(\mathrm{~s}) \stackrel{1000 \mathrm{~K}}{\longrightarrow} \mathrm{CaO}(\mathrm{s})+\mathrm{CO}_2(\mathrm{~g})\)
The main industrial source of carbon dioxide is as a by-product in the manufacture of hydrogen used in the Haber process to make ammonia.
⇒ \(\mathrm{CH}_4+2 \mathrm{H}_2 \mathrm{O} \rightleftharpoons \mathrm{CO}_2+4 \mathrm{H}_2\)
Properties and reactions of Carbon dioxide
Carbon dioxide is a colourless and odourless gas. It is slightly soluble in water. The solubility increases with an increase in pressure.
Soda water and aerated soft drinks contain carbon dioxide dissolved in water. Carbon dioxide dissolves in water to form carbonic acid, which is a weak, dibasic acid.
⇒ \(\begin{gathered}
\mathrm{CO}_2(\mathrm{~g})+\mathrm{H}_2 \mathrm{O}(\mathrm{l}) \rightarrow \underset{\text { carbonic acid }}{\mathrm{H}_2 \mathrm{CO}_3(\mathrm{aq})} \\
\mathrm{H}_2 \mathrm{CO}_3(\mathrm{aq})+\mathrm{H}_2 \mathrm{O}(\mathrm{l}) \rightleftharpoons \mathrm{H}_3 \mathrm{O}^{+}(\mathrm{aq})+\underset{\text { hydrogen carbonate }}{\mathrm{HCO}_3^{-}(\mathrm{aq})} \\
\mathrm{HCO}_3^{-}(\mathrm{aq})+\mathrm{H}_2 \mathrm{O}(\mathrm{l}) \rightleftharpoons \mathrm{H}_3 \mathrm{O}^{+}(\mathrm{aq})+\underset{\text { carbonate }}{\mathrm{CO}_3{ }^{2-}(\mathrm{aq})}
\end{gathered}\)
Thus, a solution of carbon dioxide in water is a mixture of CO2, H2CO3, HCO3– and CO32-. The dissolution in water is important biochemically as the H2CO3 /HCO3- buffer helps to maintain the pH of blood between 7.2 and 7.4.
Tire presence of carbon dioxide in the atmosphere (0.03%) is balanced by two biological processes— photosynthesis and respiration.
Carbon dioxide is used by green plants in photosynthesis to make sugar, which on combustion during respiration releases carbon dioxide.
⇒ \(6 \mathrm{CO}_2+12 \mathrm{H}_2 \mathrm{O} \stackrel{\text { sunlight }}{\longrightarrow} \underset{\text { glucose }}{\mathrm{C}_6 \mathrm{H}_{12} \mathrm{O}_6}+6 \mathrm{O}_2+6 \mathrm{H}_2 \mathrm{O}\)
\(\mathrm{C}_6 \mathrm{H}_{12} \mathrm{O}_6+6 \mathrm{O}_2 \rightarrow 6 \mathrm{CO}_2+6 \mathrm{H}_2 \mathrm{O}+\text { energy }\)Though carbon dioxide is not toxic, an excess of it in the atmosphere causes climatic changes which may have far-reaching consequences.
Increased burning of fuels and indiscriminate reduction of forest cover is responsible for the increase of the carbon dioxide level in the atmosphere. This has caused global warming (greenhouse effect).
When carbon dioxide is cooled under pressure (50-60 atm), it solidifies. The solid formed is called dry ice, which sublimes at -78°C under atmospheric pressure.
It is used as a refrigerant. Carbon dioxide, being a nonsupporter of combustion, is used in fire extinguishers, especially those meant for electrical fires.
They contain liquid carbon dioxide under pressure. Rapid vaporisation of liquid carbon dioxide is used for blasting in coal mines.
Structure Of Carbon Doxide:
Carbon dioxide has a linear structure with zero dipole moment. The carbon atom in the molecule is sp hybridised.
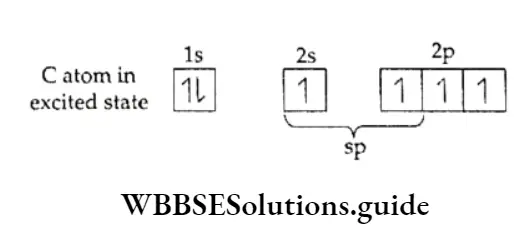
The carbon atom has two sp hybridised orbitals which form a bond with two oxygen atoms. The other two unpaired electrons on carbon form pπ-pπ bonds with oxygen. So the expected structure is: O—C=O:
However, the C —O bond length of 115 pm is less than the C—O double bond length of 122 pm. This can be explained by considering carbon dioxide to be a resonance hybrid of the following canonical forms.
⇒ \(: \overline{\mathrm{O}}-\mathrm{C} \equiv \stackrel{+}{\mathrm{O}}: \longrightarrow: \mathrm{O}=\mathrm{C}=\ddot{\mathrm{O}}: \longrightarrow:_{\mathrm{O}}^{+} \equiv \mathrm{C}-\overline{\mathrm{o}}:\)
Silicon dioxide
Silicon dioxide (SiO2), commonly known as silica, along with silicates, make up a major portion of the earth’s crust. Silica exists in both crystalline and amorphous forms.
Flint is the amorphous form of silica whereas the common crystalline forms are quartz, tridymite and cristobalite, which can be interconverted with the temperature change.
Unlike carbon dioxide, which consists of discrete molecules (due to pπ-pπ bonding) and is a gas, silicon dioxide is a high-melting solid.
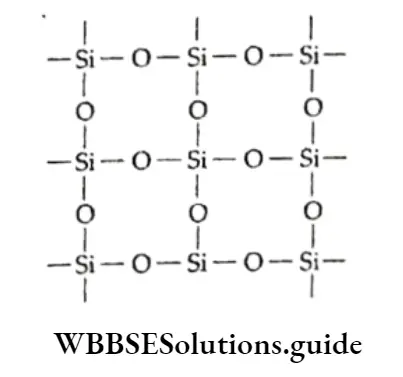
Unlike carbon, silicon cannot form a pπ-pπ bond with oxygen; in silica, each silicon atom is tetrahedrally surrounded by four covalently bonded oxygen atoms and each oxygen atom is bonded to two silicon atoms.
This structure extends in three dimensions to form a macromolecule. A lot of energy is needed to break the rigid structure and thus silica has a very high melting point Since the Si—O bond is very strong, silica is not so reactive. Being an acidic oxide, it reacts with hot alkalis and does not add.
⇒ \(\mathrm{SiO}_2+2 \mathrm{NaOH} \stackrel{\text { heat }}{\longrightarrow} \mathrm{Na}_2 \mathrm{SiO}_3+\mathrm{H}_2 \mathrm{O}\)
However, it is attacked by hydrogen fluoride
⇒ \(\mathrm{SiO}_2+4 \mathrm{HF} \longrightarrow \mathrm{SiF}_4+2 \mathrm{H}_2 \mathrm{O}\)
Quartz is used as a piezoelectric material (having the ability to generate voltage when mechanical stress is applied or dee versa) for crystals in gramophone pickups and gas lighters, and for making crystal oscillators in radios, televisions and computers.
It is also used in optical components such as lenses and prisms. Silica gel, an amorphous form of silica, is used as a catalyst in the petroleum industry, in chromatography and as a drying agent Kieselguhr, another amorphous form of silica, is used in filtration plants and as a filtration aid (e.g., dynamite contains glyceryl trinitrate and glyceryl dinitrate with Kieselguhr as adsorbent).
Silicates
Any 3 substance containing negative ions composed of silicon and oxygen is a silicate. For example, phenate (Be2SiO4) contains beryllium and beryl (Be3Al2Si16O18) contains beryllium and aluminium along with silicate ions.
A large number of silicate minerals exist in nature. The basic unit in all silicates is an sp3 hybridised silicon atom linked to four oxygen atoms, i.e., the SiO44- unit. This unit can be represented in either of the two ways.
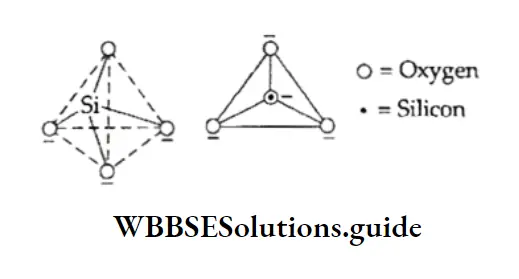
These tetrahedra may exist as discrete units or may be linked to each other by sharing comers.
This gives rise to a wide variety of silicates, which comprise rings, chains, sheets and three-dimensional structures.
Cations linked to the negatively charged silicate units help maintain the electrical neutrality of the species.
If some silicon atoms are replaced by aluminium, we get aluminosilicates. Silicates are classified based on the number of oxygen atoms shared per tetrahedron. A few representative types of silicates.
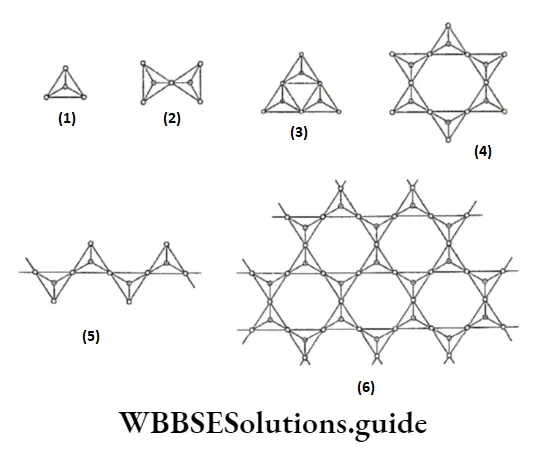
Some important two-dimensional silicates are beryl, mica and asbestos. Cement and glass are man-made silicates.
In addition to the two-dimensional silicates, three-dimensional silicates are also known. In these all four oxygen atoms of a tetrahedron are shared and some silicon atoms are replaced by aluminium, and some cations like Na+, K+ and Ca2+ are introduced to maintain electrical neutrality.
Two important three-dimensional silicates are feldspars and zeolites. Feldspars are the most important rock-forming minerals. Zeolites have an open honeycomb-like structure.
The cavities present may account for more than 50 per cent of the space by volume. The mineral is highly porous and contains water molecules in the channels.
The structure of the mineral is similar to that of a cage. Some examples of zeolites are natrolite, chabazite and sodalite. Zeolites act as molecular sieves as they allow small molecules to pass through them and retain the larger ones.
They are capable of separating straight-chain hydrocarbons from branched-chain ones and are, therefore, used in petroleum refining. They are also used as catalysts, particularly in petrochemical industries for the cracking of hydrocarbons and isomerisation.
A particular type of zeolite, ZSM-5, acts as a catalyst in the conversion of alcohol to gasoline. Zeolites have exchangeable cations, and so they are used in water-softening.
Silicones
These are a group of synthetic organo-silicon polymers. The basic unit is formed by alternating silicon and oxygen atoms with alkyl or aryl groups attached to the silicon. They are represented by the general formula (R22SiO)n or
![]()
where R is an alkyl or aryl group. The compounds contain Si—C bonds, which are almost as strong as C—C bonds.
Since the empirical formula R3SiO is similar to that of a ketone (R2CO), these materials are named silicones. They may be linear, cyclic or cross-linked.
The starting materials for the manufacture of silicones are alkyl- or arylsubstituted silicon chlorides (RnSiCl4-n), where R is the alkyl or aryl group.
When methyl chloride is treated with silicon in the presence of copper powder (catalyst) at 570 K, a mixture of methyl-substituted chlorosilanes — CH3SiCl3, (CH3)2SiCl2, (CH3)3SiCl, along with, a small amount of (CH3)4Si, is formed. The products can be separated by fractional distillation.
The hydrolysis of dimethyl dichlorosilane yields a straight-chain polymer. During hydrolysis, the chlorine atoms are replaced by —OH groups to give silanols (similar to alcohols).
![]()
The silanols undergo intermolecular condensation to give a polymer.
![]()
The chain length of the polymer can be controlled by adding a little (CH3)3SiCl. This on hydrolysis gives (CH3)3Si—OH. This has only one —OH group, which attaches itself to one end of the chain, thereby limiting the chain size.
Similarly, the addition of CH3SiCl3 to (CH3)2SiCl2 during hydrolysis gives a cross-linked polymer. Commercially useful polymers are generally methyl or phenyl derivatives. Silicones may be used in the form of oils, rubbery elastomers or resins.
They have unique properties like chemical inertness, thermal stability and electrical insulation.
Owing to these properties silicones are used for making electrical insulators and nonstick utensils.
Since they are surrounded by nonpolar, hydrophobic organic groups which repel water, silicones are used for the waterproofing of textiles. They are also used as greases, varnishes and antifoam agents in breweries and sewage disposal plants.
Other uses of silicones include applications in surgical and cosmetic implants due to their biocompatible nature.
![]()
The p- Block Elements Multiple Choice Questions
Question 1. Which among the following shows the most stable +2 oxidation state?
- Al
- Tl
- Si
- Pb
Answer: 4. Pb
Question 2. The hybridisation of boron in BF3 is
- Sp
- Sp2
- Sp3
- Dsp2
Answer: 2. Sp2
Question 3. Which of these is not a Lewis acid?
- BF3
- AlCl3
- CCl4
- Bcl3
Answer: 3. CCl4
Question 4. [SiF6]2- is known but [CF6]2- is not known because carbon
- Is very electronegative
- Has small size
- Does not have d orbitaLs
- None of these
Answer: 3. Does not have d orbitals
Question 5. A1C13 ionises in an aqueous solution because
- It is a Lewis acid
- It is an ionic compound
- It is unstable
- Its hydration enthalpy exceeds its lattice enthalpy
Answer: 4. Its hydration enthalpy exceeds its lattice enthalpy
Question 6. Which gas is evolved when aluminium is treated with NaOFI?
- H2
- O2
- Both O2
- and H2
- Water vapour
Answer: 1. H2
Question 7. Thermodynamically, the most stable form of carbon is
- Diamond
- Graphite
- Fullerene
- Coke
Answer: 2. Graphite
Question 8. Pb(IV) compounds are
- Stable
- Oxidising
- Reducing
- Not Known
Answer: 2. Oxidising
Question 9. Which of these is an acidic oxide?
- SiO2
- pbO2
- SnO2
- PbO
Answer: 1. SiO2
Question 10. Aluminium chloride exists as a
- Dimer
- Monomer
- Trimer
- Tetramer
Answer: 1. Dimmer
“p-Block elements, periodic table group, properties, and functions”
Question 11. B(OH)3 is a
- Base
- Monobasic Acid
- Dibasic acid
- Tribasic acid
Answer: 2. Monabasic Acid
Question 12. Which of these is electron-deficient?
- SnCl2
- SnCl4
- CC14
- AlCl3
Answer: 4. AlCl3
Question 13. Which of these is the most stable?
- PbCl2
- SnCl2
- SiCl2
- CC12
Answer: 1. PbCl2
Question 14. Carbon forms several compounds because of its
- Variable valency
- Property of catenation
- High electronegativity
- High ionisation enthalpy
Answer: 2. Property of catenation
Question 15. Which of these is the least stable?
- Pbl4
- PbI4
- SnCl2
- SnCl4
Answer: 2. PbI4
Question 16. Which of these is an electron-precise molecule?
- BF3
- CCL4
- PbCl2
- AlCl3
Answer: 2. CCL4
Question 17. Which among the following is expected to be oxidising?
- CC14
- SiCl4
- SnCl4
- PbCl4
Answer: 4. PbCl4
Question 18. Which among the following is a neutral oxide?
- Pbo
- A12O2
- CO
- CO2
Answer: 3. CO
Question 19. Which of these is an important constituent of many fuels?
- CO
- CO2
- SiO2
- None Of These
Answer: 1. CO
