The d-and f-block Elements
The d block of the periodic table lies between the s and the p block. It comprises rows which are conveniently referred to as series. The first three rows or series are formed by the progressive filling of 3d, 4d and 5d orbitals respectively. The first row of the d block is Period 4, known as the 3d series and so on. The fourth row, which begins with the filling of the 6d series is still incomplete.μ
The terms d-block elements and transition elements are used interchangeably. However, they do not mean the same thing. Transition elements are those which have an incomplete d orbital or those which give rise to cations having partially filled d subshells. Thus elements of Group 12—Zn, Cd and Hg—are d-block elements but not transition elements.
The f block comprises elements in which 4f and 5f orbitals are progressively filled. The members of this block are actually part of Group 3. The two series of the flock are known as lanthanoids and actinoids respectively.
In the case of the d-block elements, the last electron enters the penultimate orbital whereas in the f-block elements (also called inner transition elements), the last electron enters the ante-penultimate orbital, i.e., the f orbital.

The transition and inner transition elements are thus characterised by the presence of partially filled d and f orbitals respectively. This confers some specific properties of those elements and merits a separate study of them along with some of their compounds. However, the normal rules of valence, as applicable to the main group elements, can be used here too.
The d-block Elements
The transition elements exhibit certain characteristic properties. They are all metals exhibiting high tensile strength, malleability and ductility. They are excellent conductors of heat and electricity and have lustre.
They have high melting and boiling points, exhibit variable oxidation states and paramagnetic behaviour in many compounds and form coloured compounds. They form complexes with different anions and neutral species and may also form alloys and interstitial compounds with other metals.
Many transition metals and their compounds display catalytic properties. Some of the transition metals exhibiting variable valency form unstable intermediate compounds. Also, some transition metals provide a suitable reaction surface.
The lattice structures of transition metals vary. The predominant structures displayed are hexagonal close-packed, cubic close-packed, body-centred cubic, etc. Mercury, being the exception, is a liquid at room temperature.
Electronic Configuration
The general electronic configuration of d-block elements is (n- 1)d1-10ns1-2. There may be one or two electrons in the s orbital of the outermost shell and one to ten electrons in the d orbitals of the inner shell (n – 1). There are exceptions to these generalizations which arise due to very little difference in the energies of (n -l)d and ns orbitals.
In addition to this, the half-filled or completely filled subshells are relatively more stable. Consequently, the electronic configurations of Cr and Cu are 3d54s1 (not 3d44s2) and 3d104s1 (not 3d94s2) respectively. There are also many anomalies in the electronic configurations of the elements in the 4d and 5d series.
The sequence of filling up of orbitals according to the energy levels in multi-electron atoms not only depends on the electron-nuclear attraction, but also on inter-electron repulsion, but a detailed discussion is beyond the scope of this presentation. The outer electronic configurations of the d-block elements are shown in.
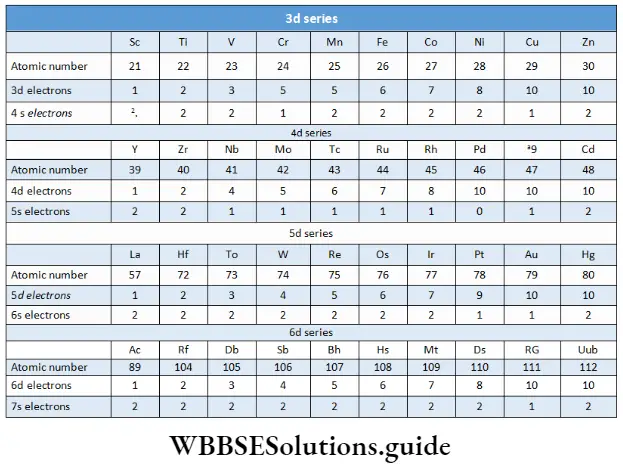
The electronic configurations of zinc, cadmium and mercury (which are also the last members of the three respective series) are represented as (n- 1)d10ns2. As already stated, they are not regarded as transfer dozens since the d orbitals are completely filled in the ground state and also in their common oxidation state The d-block elements display certain characteristic properties, which we will study in this chapter- Yo also observes a high degree of similarity in the properties of d-block elements horizontally, in contrast to those s- and p-block elements, where elements of the group display similarities in properties vertically However Group similarities do existing d-block elements also.
Atomic And Ionic Radii

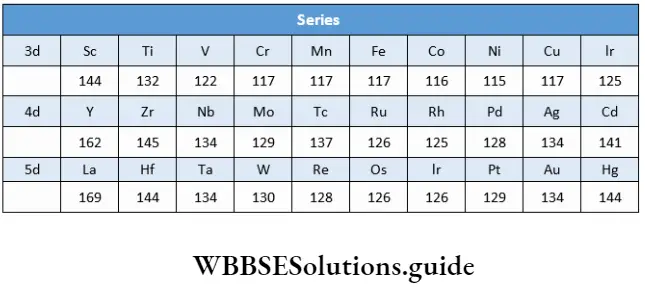
We shall discuss the trends in atomic and ionic sizes across the period and down the group. The size of the atom initially decreases across a period after which there is no appreciable change and finally at the end of the series there is an increase in size.
On moving across a period in the periodic table, the nuclear charge increases and the size of the atom decreases. In the case of the transition elements, each time the nuclear charge increases by unity the extra electron enters the d orbital.
The d-orbital electrons screen the outermost s-orbital electrons from the nucleus incompletely because of their relatively less efficient shielding power, in comparison to that of the electrons in s- and p-orbitals.
As the number of d electrons increases across the period, the screening effect also increases and this, to some extent, counteracts the effect of increased nuclear charge. This is prominent in the middle of the series and thus in the region, a fairly constant size is observed.
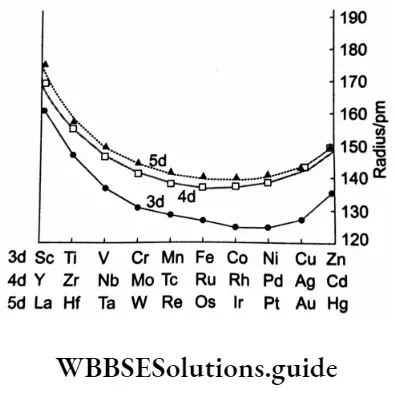
As you know, the 3d series constitutes elements with atomic numbers 21 – 30, In the series, electron pairing in d orbitals is not observed till Mn (atomic number = 25). So electron-electron repulsions are not significant.
The pairing of electrons starts from Fe (atomic number = 26) and this brings about inter-electron repulsions which cause the electron cloud to expand resulting in an increase in atomic size, This effect works in opposition to the effect of increased nuclear charge, which tends to reduce the size, and the atomic radii remain practically the same after Cr.
The atomic size of elements increases down the group as extra shells of electrons are added. This trend is observed strictly for Sc, Y and La (Group 3). However, in the subsequent groups, an increase in size is observed between the corresponding members of the 3d and 4d series, but there is hardly any increase between the 4d and 5d series.
There are 14 lanthanoid elements between La and Hf in the 5d series. They all have two outermost s electrons and are classified together because an increase in the proton number corresponds to an increase in the number of 4f electrons (here the antepenultimate 4f shell is filled).
There is a gradual decrease in the atomic size of the lanthanoid elements and this is called lanthanoid contraction. This cancels the normal increase in atomic size on moving down a group. As a result the elements of 4d- and 5d-series have similar sizes and display similar properties.
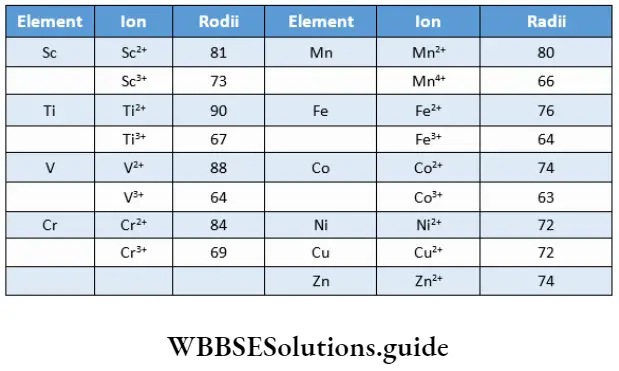
For a given charge the ionic radius decreases slowly with the increase in atomic number. The ionic radii decrease with an increase in oxidation state, as the nuclear charge increases.
Density
The d-block elements have high density. The atomic volumes of these elements are low as compared to those of the elements of groups 1 and 2. Thus, the densities of the transition elements are high and generally exceed 5 gcm-3 except for Sc (3.0 g cm-3), Y and Ti (4.5 gems-3). The densities of the elements of the 4d and 5d series are very high, for example, osmium and iridium have densities of 22.57 g cm-3 and 22.61 g cm-3 respectively.
Melting And Boiling Points
The melting and boiling points of the transition elements are very high. Most of them melt above 1273 K and the elements Ta, W and Re melt above 3273 K. Notable exceptions are Zn (693 K), Cd (594 K) and Hg which is a liquid at room temperature.
The melting points of transition metals are high because of unpaired d electrons which participate in metallic bond formation. In any row, metals with d5 configuration (exceptions Mn, Tc) have high melting points, indicating that a large number of unpaired d electrons give rise to strong metallic bonds.
The great strengths of the metal-metal bonds are also manifested in high enthalpies of atomisation. In zinc, cadmium and mercury the d shell is completely filled and the d electrons do not participate in bond formation.
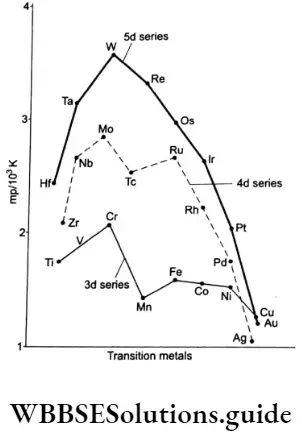
Ionisation Enthalpy
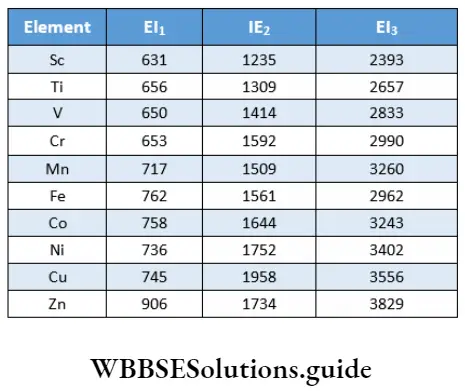
A comparison of ionisation enthalpies of elements gives an idea of the ease with which they form ions, or how electropositive they are. Transition elements have high ionisation enthalpies due to the small size of the atoms and high nuclear charge. The ionisation enthalpies of the first series of transition elements are given in.
Generally, ionisation enthalpy increases across a period. However, some deviations are observed from this trend. On moving across a period, due to the increase in nuclear charge, the nucleus tends to pull the outermost electrons inwards.
In transition elements, the electrons are filled in the penultimate shell (n-l)d. The d subshell shields the outermost, ns electrons from the pull of the nucleus. Thus the effect of increased nuclear charge is nullified to an extent by the screening effect of the inner d-orbital electrons and consequently, the ionisation enthalpy increases only gradually across a series. There is a sudden increase in the first ionisation enthalpy of zinc.
This is due to its stable outermost electronic configuration—3d10 4s2. The other exceptions are chromium and copper whose second ionisation enthalpies are notably higher than those of their neighbours.
This is because of the stable outer electronic configuration of Cr+(3d5) and Cu+(3d10) so the removal of an electron becomes difficult. As you already know, half-filled and completely filled orbitals are stable.
Apart from these exceptions, the values in Table 8.4 show that there is a similar trend of increase in the first and the second ionisation enthalpies across the period. The third ionisation enthalpies are, however, quite high and there is a deviation from the normal trend in the case of Mn2+ and Fe2+.
The third ionisation enthalpy of manganese is high as Mn2+ has a stable d5 configuration. Also, the high values of third ionisation enthalpies of nickel, copper and zinc indicate that the highest oxidation state attainable for these is two.
Oxidation States
One of the most striking features of transition elements is that they exist in several oxidation states, which change in units of one. For example, iron exists as Fe2+ and Fe3+ and copper as Cu+ and Cu2+.
In contrast, the oxidation states of p-block elements differ by units of two, for example, Sn22+ Sn4+ (inert pair effect). The common oxidation states of the elements of the 3d series are shown in.
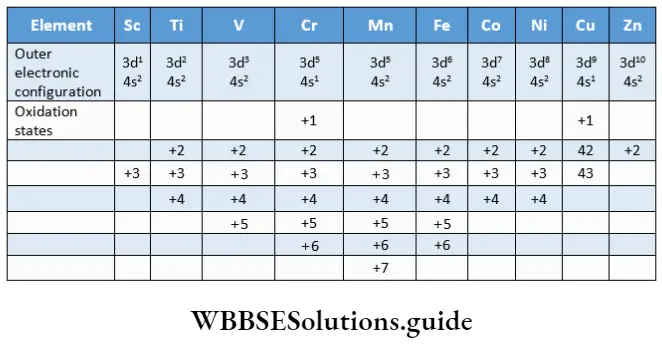
Transition elements exhibit variable oxidation states in their compounds as both (n-l)d and ns electrons of atoms participate in bond formation. As you can see in up to manganese (starting from titanium across the series) the lowest oxidation state of an element is equal to the number of s electrons and the highest oxidation state is equal to the sum of the number of s and d electrons.
Once we go beyond the d5 electronic configuration in the series, the tendency of all d electrons to participate in bond formation decreases. This is because as the number of d electrons increases, they tend to pair up, leaving fewer orbitals available for the sharing of electrons.
The number of oxidation states exhibited by the respective elements is less at both the extreme ends of the series. At the top end (Sc, Ti) there are few electrons to lose or share during bond formation.
The higher oxidation states exist in the form of fluorides, oxides and oxoanions (MnO4–, CrO42-). In addition to this, some elements exhibit zero and negative oxidation states with some ligands like carbon monoxide. For example, in Fe(CO)5, Ni(CO)4 and Co2(CO)8 the metals exhibit zero oxidation state, whereas the oxidation state of cobalt in Na2[Co(CO)4]is negative.
Stability of the various oxidation states
Compounds are regarded as stable if they exist at room temperature and remain unaffected by air and water. In general higher oxidation states are more stable for the elements of the 4d and 5d series in comparison to those of the 3d series.
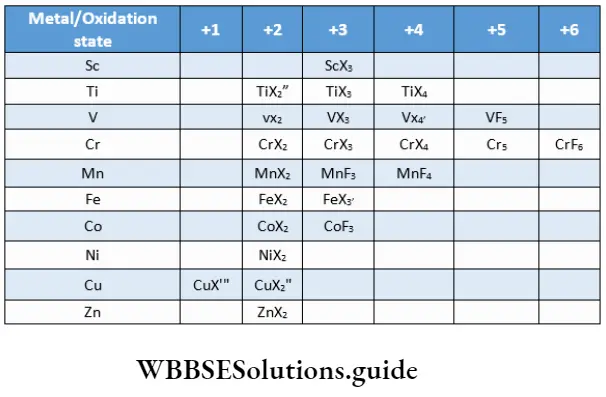
For example, CrO3 (3d series) is unstable and therefore strongly oxidising whereas MoO3(4d series) and WO3 (5d series) are quite stable. The halides and oxides formed by the elements of the 3d series are shown in. A few interesting observations can be made.
Note:
- X denotes all halides.
- X’ denotes fluoride, chloride, bromide.
- X” denotes fluoride, chloride.
- X”‘ denotes chloride, bromide, iodide
It may be noted that the highest oxidation state for titanium is represented by all halides whereas for vanadium and chromium, it is represented in only fluorides. Fluorine is able to stabilise higher oxidation states because it is highly electronegative and the M-F bond has high bond enthalpy.
However, fluorides in low oxidation states are not known. This is presumably because fluorine is highly oxidising. Oxohalides of the type VOX3 and MnO3F (representing the metal in the highest oxidation state) are known.
Transition metals in stable oxidation states from all halides. Those in strongly reducing states form heavier halides (not fluorides), whereas those in strongly oxidising states do not form iodides. For example, Cul is known but not CuI2 as Cu2+ oxidises I– to I2
⇒\(2 \mathrm{Cu}^{2+}+4 \mathrm{I}^{-} \rightarrow \mathrm{Cu}_2 \mathrm{I}_2+\mathrm{I}_2\)
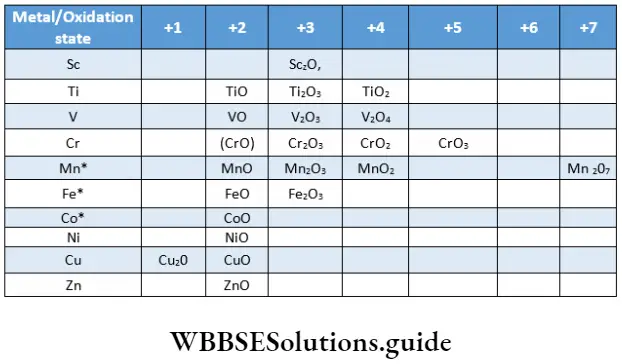
Note: Mixed oxides are also known—Mn3O4, Fe3O4, Co3O4
As you can see, oxides in the highest oxidation states of various metals are known, for example, Sc2O3, TiO2, V2O5, CrO3, Mn2O7. Beyond manganese, the stability of the higher oxidation state decreases. In addition to oxides, oxoanions in high oxidation states are also known, for example VO43-, CrO43-, MnO42-and MnO4–.
Sometimes the magnitude of ionisation enthalpies gives an idea of the relative stabilities of oxidation states. This can be well understood by considering the case of nickel and platinum. Pt(4) species are more stable than Ni(4), but Ni (2) species are more stable than Pt(2). The ionisation enthalpies of the metals are as follows.
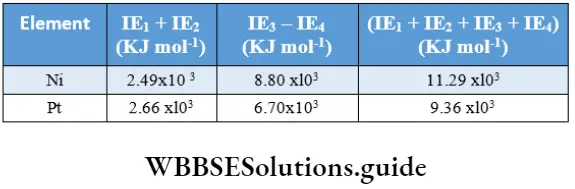
Since the sum of the first two ionisation enthalpies of nickel is less than that of platinum, Ni(2) compounds are more stable. However, since the sum of the first four ionisation enthalpies of platinum is less than that of nickel Pt(4) species are more stable.
Standard Electrode Potentials, E°
The standard electrode potentials (Eθ) of the elements of the 3d series for the M2+/M couple are shown.
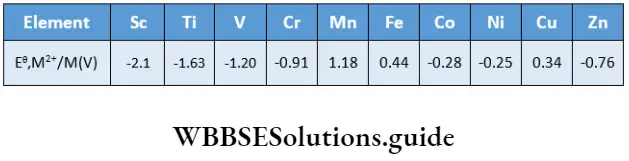
As you can see, except copper all elements have negative Eθ& values, i.e., they can liberate hydrogen from acids and in the process themselves get oxidised, though the rate at which these metals are oxidised is sometimes slow.
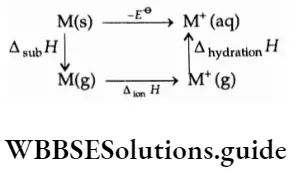
However, metals like titanium and vanadium are passive to dilute nonoxidising acids. The standard electrode potential values are related to various thermochemical parameters like enthalpy of sublimation, ionisation enthalpy and enthalpy of hydration. The relationship between these can be shown in the cycle depicted below.
The Eθ values indicate a reduced tendency to form M2+ species across the series. This is attributed to increasing first and second ionisation enthalpy values across the series. The Eθ values of manganese, nickel and zinc are more negative than expected.
This is due to the extra stability of half-filled (d5 ) and completely filled (d10) configurations of Mn2+ and Zn2+ respectively and a high enthalpy of hydration of Ni2+. The positive Eθ value for copper could be attributed to high ionisation enthalpy and low hydration enthalpy.
The Eθ values for M3+/M2+ redox couple for Mn3+/Mn2+ (1.57 V), Fe3+/Fe2+ (0.77 V) and Co3+ /Co22+ (1.97 V) are positive whereas those for Ti3+/Ti2+ (-0.37 V), V3+ / V2+ (-0.26 V) and Mn3+/Mn2+ (-0.41 V) are negative. This indicates that Mn3+, Co3+ and Fe3+ are oxidising andTi2+, V2+ and Cr2+ are reducing.
Formation Of Coloured Species
Many ionic and covalent compounds of transition metals are coloured. lists the colours of the ions in aqueous solutions where water molecules are ligands.
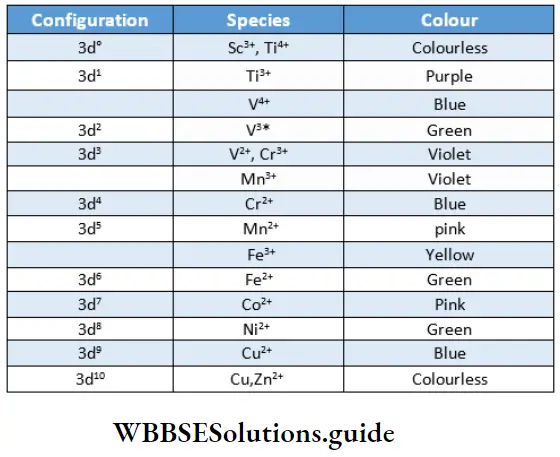
In contrast, compounds of the s- and p-block elements are generally white or colourless except for a few in which colour arises due to the anion, for example, KMnO4, K2CrO4, K2Cr2O7 or where other factors like charge transfer, polarisation, etc., come into play. For example, Agl and SnI4 are coloured. Sometimes colour arises due to nonstoichiometry.
When light passes through a material, a part of it is absorbed. If the frequency of the light absorbed is in the visible region, the transmitted light is coloured and the colour observed is complementary to the colour absorbed. The colour of transition metals arises due to incomplete 3d subshells.
The d orbitals of an isolated gaseous atom are degenerate. This degeneracy is lifted when the metal ion is surrounded by anions or a solvent. This is because the surrounding groups affect the energy of some d orbitals more than the others so two groups of d orbitals are formed with different energies.
This is called crystal field splitting. Due to this splitting of orbitals an electron may move from the set of lower-energy d orbitals to the set of higher-energy d orbitals by absorbing minute energy.
Such transitions are called d-d transitions and are mainly responsible for the colour of the compounds, d-d transitions are not possible in d° and d10 configurations and therefore Sc3+ (d0), Ti4+ (d0), Cu’ (d10) and Zn2’ (d10) are colourless.
Magnetic Properties
When different materials are placed in a magnetic field they exhibit different characteristics. The two main types of magnetic behaviour are paramagnetism and diamagnetism. Substances which are attracted by the applied magnetic field are paramagnetic whereas those repelled by it are diamagnetic. The magnetic lines of force travel more readily through paramagnetic substances than through a vacuum.
Paramagnetism arises as a result of unpaired electron spins in the atom. Therefore, substances having unpaired electrons are paramagnetic. Many transition metals and their compounds exhibit paramagnetism.
The extent of paramagnetic character is determined by the magnetic moment of a substance which is determined experimentally and expressed in Bohr magneton (BM) units. The magnetic moment of a substance is the sum of the magnetic moments of each of the unpaired electrons.
The magnetic moment of an electron is associated with its spin angular momentum (produced due to its spinning on the axis) and orbital angular momentum (produced due to its motion in the orbital).
The total magnetic moment is called the effective magnetic moment (μeff). Generally, the spin contribution outweighs or quenches the orbital contribution. The magnetic moment due to spin (|ispmoniy ) is related to the number of unpaired electrons (n) by the ‘spin only’ formula.
⇒\(\mu_{\mathrm{eff}} \approx \mu_{\mathrm{spin} \text { only }}=\sqrt{n(n+2)}\)
In a diamagnetic substance, since all electrons are paired, the magnetic moment is zero.
The magnetic moment increases as the number of unpaired electrons increases. Therefore, the observed magnetic moment of an atom, molecule or ion may give an idea of the number of unpaired electrons present. A single 1 s electron has a magnetic moment of 1.73 Bohr magnetons (BM).
Iron, cobalt and nickel are ferromagnetic. This is an extreme case of paramagnetism. Such substances are very strongly attracted by magnets and can be magnetised.
Catalytic Properties
Many transition metals and their compounds exhibit catalytic properties. Some examples are as follows.
- Titanium(4) chloride is used as the Natta catalyst in the production of polythene. The compound,s generally used along with triethylaluminium and is called the Ziegler-Natta catalyst.
- Finely divided iron is used in the Haber process for the manufacture of ammonia.
- Finely divided nickel is used for the hydrogenation of fats and oils.
- Copper is used in the manufacture of alkylchlorosilanes, for example(CH3)2SiCI2, which is the starting material for the preparation of a class of industrially important polymers—silicones.
- Platinum is used in the Ostwald process for the catalytic oxidation of ammonia to nitric oxide.
- V(5) oxide is used in the contact process for the manufacture of sulphuric acid.
Transition metals and their compounds serve as effective catalysts because the metal ions can change their oxidation states, forming unstable intermediate compounds.
For example, in the oxidation of SO2 to SO3 V(V) oxide, the catalyst reacts with SO2 to form SO3 and the unstable V(4) oxide, which reacts with oxygen to regenerate the V(5) oxide.
The reaction may be represented as
⇒\(\begin{aligned}
& 2 \mathrm{~V}_2 \mathrm{O}_5+2 \mathrm{SO}_2 \longrightarrow 2 \mathrm{~V}_2 \mathrm{O}_4+2 \mathrm{SO}_3 \\
& 2 \mathrm{~V}_2 \mathrm{O}_4+\mathrm{O}_2 \longrightarrow 2 \mathrm{~V}_2 \mathrm{O}_5 \\
& \hline 2 \mathrm{SO}_2+\mathrm{O}_2 \stackrel{\mathrm{V}_2 \mathrm{O}_5}{\longrightarrow} 2 \mathrm{SO}_3 \quad \text { (overall reaction) }
\end{aligned}\)
Another example is the reaction between iodide and persulphate ions, which is catalysed by the change in the oxidation state of iron.
⇒\(2 \mathrm{I}^{-}+\mathrm{S}_2 \mathrm{O}_8^{2-} \stackrel{\mathrm{Fe}^{3+}}{\longrightarrow} \mathrm{I}_2+2 \mathrm{SO}_4^{2-}\)
The catalytic action of iron is explained as
⇒\(2 \mathrm{Fe}^{3+}+2 \mathrm{I}^{-} \longrightarrow 2 \mathrm{Fe}^{2+}+\mathrm{I}_2\)
⇒\(2 \mathrm{Fe}^{2+}+\mathrm{S}_2 \mathrm{O}_8^{2-} \longrightarrow 2 \mathrm{Fe}^{3+}+2 \mathrm{SO}_4^{2-}\)
In addition to this, transition metals also provide a large surface area for the reactants to be absorbed by the formation of bonds between reactant molecules and surface atoms of the catalyst utilising 3d and 4s electrons (in the case of 3d series of transition metals).
This increases the concentration of the reactant molecules on the surface of the catalyst and also lowers the activation energy by weakening the bonds in the reactant molecules.
Tendency To Form Complexes
Transition metals form a unique class of complex compounds. In such compounds, the central metal ion (cation) is linked by coordinate bonds to species which can donate an electron pair. These are called ligands, and they may be anions or neutral molecules containing unshared electron pairs.
The transition elements have a strong tendency to form complexes because of the small size of their metal ions, high ionic charges and the availability of vacant d orbitals of the right energy to accept lone pairs of electrons from ligands. The metal ion utilises(n-l)d orbitals for the bond formation with the ligands.
Tendency To Form Interstitial Compounds
Transition metals form interstitial compounds when small atoms like boron, nitrogen and hydrogen are trapped in empty spaces or interstices in the crystal lattices of metals. Such substances are hard, but less malleable and ductile than pure metals.
Their melting and boiling points are higher than those of the pure metal, the compounds retain metallic conductivity and are chemically inert. They may be stoichiometric as well as nonstoichiometric.
Most metallic carbides of d-block elements are interstitial compounds where certain atoms occupy octahedral voids in the metallic lattice. Some of these are economically useful materials. For example, tungsten carbide (WC) is used for cutting tools and cementite (Fe3C) is a major constituent of steel and cast iron.
Tendency To Form Alloys
The atomic radii of transition metals are comparable. Therefore, it is possible to replace one metal with another in the crystal lattice. This results in the formation of alloys which are actually a blend of metals (atoms of one metal distributed randomly among those of the other in the lattice).
Alloys are harder and more resistant to corrosion than the individual metals. The common ones are ferrous alloys. Chromium, vanadium, manganese, tungsten and molybdenum are used in the production of stainless steel and other varieties of steel.
Alloys of transition metals with nontransition metals, for example, brass (an alloy of copper and zinc) and bronze (an alloy of copper and tin) are important industrial processes.
Comparison of metals of 3d series
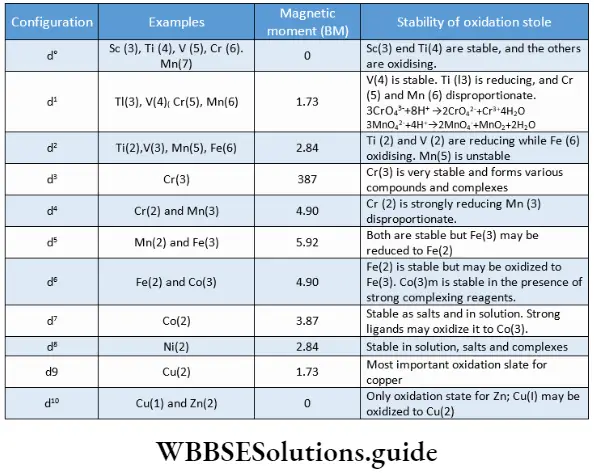
The transition metals of the first row display certain similarities. These metals exhibit variable oxidation states. Therefore, it is possible that different metals in various oxidation states show identical electronic configurations.
This results in similarity in certain properties of the metals like colour and magnetic behaviour. A comparison of the metals of the 3d series on the basis of their d-electron. configuration is depicted.
Some Important Compounds of Transition Metals
Oxides
A large number of oxides are known as transition metals due to their variable oxidation states. You already have an idea about the vast number of oxides formed. In general, metals in the higher oxidation states form acidic and covalent oxides while those in the lower oxidation states form basic and ionic oxides. The oxides formed in the intermediate oxidation states of metals are amphoteric.
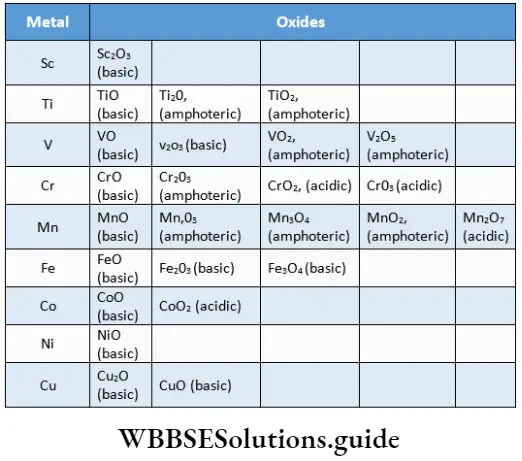
From Group 3 to Group 7 the highest oxidation state in the respective oxide is the same as the group number. Thus, the highest oxidation state is attained in Sc2O3, TiO2, V2O5, CrO3 and Mn2O7. Beyond Group 7, the stability of the highest oxidation state decreases and the highest oxide of iron is Fe2O3.
In the transition metals up to those of Group 7, the highest oxidation state is also represented in the form of oxocations (VO2+, TiO2+) and oxoanions (CrO42-, MnO4–).
Potassium Dichromate (K2Cr2O7
Preparation
It is obtained from the chromite ore (FeO Cr2O3 or FeCr2O4). The first step involves the fusion of the ore with sodium carbonate in an excess of air, whereby sodium chromate is formed.
⇒ \(4 \mathrm{FeCr}_2 \mathrm{O}_4+8 \mathrm{Na}_2 \mathrm{CO}_3+7 \mathrm{O}_2 \rightarrow 8 \mathrm{Na}_2 \mathrm{CrO}_4+2 \mathrm{Fe}_2 \mathrm{O}_3+8 \mathrm{CO}_2\)
The fused mass is extracted with water in which sodium chromate dissolves, forming a yellow solution. The solution is then treated with dilute sulphuric acid, and sodium chromate is converted to sodium dichromate.
⇒ \(2 \mathrm{Na}_2 \mathrm{CrO}_4+\mathrm{H}_2 \mathrm{SO}_4 \rightarrow \mathrm{Na}_2 \mathrm{Cr}_2 \mathrm{O}_7+\mathrm{Na}_2 \mathrm{SO}_4+\mathrm{H}_2 \mathrm{O}\)
On concentrating the solution, sodium sulphate, being less soluble, crystallises out. It is filtered, the solution is further concentrated, and potassium chloride is added to obtain potassium dichromate.
⇒ \(\mathrm{Na}_2 \mathrm{Cr}_2 \mathrm{O}_7+2 \mathrm{KCl} \rightarrow \mathrm{K}_2 \mathrm{Cr}_2 \mathrm{O}_7+2 \mathrm{NaCl}\)
Since sodium dichromate is more soluble than potassium dichromate, on increasing the concentration of the solution, the orange crystals of potassium dichromate are obtained.
Properties
Potassium dichromate is an orange-red solid (melting point 671 K). It is moderately soluble in cold water. When an alkali is added to an aqueous solution of potassium dichromate, the colour changes from orange to yellow due to the formation of potassium chromate.
⇒ \(\mathrm{K}_2 \mathrm{Cr}_2 \mathrm{O}_7+2 \mathrm{KOH} \rightarrow 2 \mathrm{~K}_2 \mathrm{CrO}_4+\mathrm{H}_2 \mathrm{O}\)
⇒ \(\mathrm{Cr}_2 \mathrm{O}_7^{2-}+2 \mathrm{OH}^{-} \rightarrow 2 \mathrm{CrO}_4^{2-}+\mathrm{H}_2 \mathrm{O}\)
Acidification of the chromate solution leads to the formation of potassium dichromate and the colour changes again from yellow to orange.
⇒ \(2 \mathrm{~K}_2 \mathrm{CrO}_4+\mathrm{H}_2 \mathrm{SO}_4 \rightarrow \mathrm{K}_2 \mathrm{Cr}_2 \mathrm{O}_7+\mathrm{K}_2 \mathrm{SO}_4+\mathrm{H}_2 \mathrm{O}\)
⇒ \(2 \mathrm{CrO}_4^{2-}+2 \mathrm{H}^{+} \rightarrow \mathrm{Cr}_2 \mathrm{O}_7^{2-}+\mathrm{H}_2 \mathrm{O}\)
Hence, there is a pH-dependent equilibrium between dichromate and chromate.
⇒ \(\mathrm{Cr}_2 \mathrm{O}_7^{2-}+\mathrm{H}_2 \rightleftharpoons 2 \mathrm{CrO}_4^{2-}+2 \mathrm{H}^{+}\)
The oxidation state of chromium in chromate and dichromate is the same.
Potassium dichromate is a powerful oxidising agent in an acidic medium.
⇒ \(\mathrm{Cr}_2 \mathrm{O}_7^{2-}+14 \mathrm{H}^{+}+6 \mathrm{e}^{-} \rightarrow 2 \mathrm{Cr}^{3+}+7 \mathrm{H}_2 \mathrm{O} \quad\left(E^{\Theta}=1.33 \mathrm{~V}\right)\)
Acidified potassium dichromate can oxidise iodide to iodine, hydrogen sulphide to sulphur, iron(II) to iron(m) and tin(II) to tin(IV).
The half-reactions for these examples may be given as follows.
2I–→I2+2e–
H2S→S+2H++2e–
Fe2+→Fe3++2e–
Sn2+→ Sn4++2e–
The complete ionic equation may be obtained by multiplying the half equations for reducing agents with suitable integers and adding to the half equations of the oxidising agent such that the number of electrons cancels out.
⇒ \(\mathrm{Cr}_2 \mathrm{O}_7^{2-}+14 \mathrm{H}^{+}+6 \mathrm{I}^{-} \rightarrow 2 \mathrm{Cr}^{3+}+3 \mathrm{I}_2+7 \mathrm{H}_2 \mathrm{O}\)
Sodium dichromate is also a very powerful oxidising agent and is highly soluble in water. It is widely used in organic synthesis.
Structure
The structures of the chromate ion (CrO42 ) and the dichromate (Cr2O72 ) are shown below.
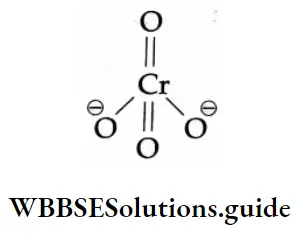
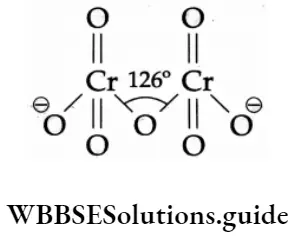
In a chromate ion, the chromium atom is tetrahedrally surrounded by four oxygen atoms. In a dichromate ion, two such tetrahedrons are linked through an oxygen atom.
Uses
Potassium dichromate is widely used as a primary standard in volumetric analysis (the sodium salt is unsuitable as it is deliquescent). It is used in the leather industry for tanning and in textile dyeing. Potassium dichromate is also employed as an oxidising agent in organic and inorganic synthesis.
Potassium Permanganate (KMnO4)
Preparation
It is prepared commercially by the fusion of the mineral, pyrolusite (MnO2), with potassium carbonate or potassium hydroxide in the presence of air. Sometimes an oxidising agent like potassium nitrate or potassium chlorate is added to aid the oxidation process. During oxidative fusion, MnO2 gets oxidised to potassium manganate, which is green in colour.
⇒ \(2 \mathrm{MnO}_2+4 \mathrm{KOH}+\mathrm{O}_2 \rightarrow 2 \mathrm{~K}_2 \mathrm{MnO}_4+2 \mathrm{H}_2 \mathrm{O}\)
The fused mass is then extracted with water and the green solution is treated with chlorine, ozone or carbon dioxide to convert manganate into permanganate.
⇒ \(2 \mathrm{~K}_2 \mathrm{MnO}_4+\mathrm{Cl}_2 \rightarrow 2 \mathrm{KMnO}_4+2 \mathrm{KCl}\)
⇒ \(2 \mathrm{~K}_2 \mathrm{MnO}_4+\mathrm{O}_3+\mathrm{H}_2 \mathrm{O} \rightarrow 2 \mathrm{KMnO}_4+2 \mathrm{KOH}+\mathrm{O}_2\)
⇒ \(3 \mathrm{~K}_2 \mathrm{MnO}_4+2 \mathrm{CO}_2 \rightarrow 2 \mathrm{KMnO}_4+2 \mathrm{~K}_2 \mathrm{CO}_3+\mathrm{MnO}_2\)
Alternatively, the green solution of potassium manganate is allowed to undergo disproportionation in an acidic or neutral medium.
⇒ \(3 \mathrm{~K}_2 \mathrm{MnO}_4+4 \mathrm{H}^{+} \rightarrow 2 \mathrm{KMnO}_4+\mathrm{MnO}_2+2 \mathrm{H}_2 \mathrm{O}+4 \mathrm{~K}^{+}\)
The oxidation of manganate to permanganate can also be carried out electrolytically using a nickel anode and an iron cathode. The cathodic and anodic compartments are separated by a diaphragm. The K2MnO4 solution is put in the cathodic compartment and potassium hydroxide in the other. During electrolysis, the manganate ion undergoes oxidation at the anode to give the permanganate ion.
⇒ \(
\underset{\text { Manganate ion }}{\mathrm{MnO}_4^{2-}} \rightarrow \underset{\text { Permanganate ion }}{\mathrm{MnO}_4^{-}}+\mathrm{e}^{-}\)
⇒ \(\text { Cathode: } 2 \mathrm{H}^{+} \text {(from water) }+2 \mathrm{e}^{-} \rightarrow \mathrm{H}_2\)
Potassium permanganate can be prepared in the laboratory by the oxidation of a manganese(II) salt by potassium peroxodisulphate.
⇒ \(2 \mathrm{Mn}^{2+}+5 \mathrm{~S}_2 \mathrm{O}_8^{2-}+8 \mathrm{H}_2 \mathrm{O} \rightarrow 2 \mathrm{MnO}_4^{-}+10 \mathrm{SO}_4^{2-}+16 \mathrm{H}^{+}\)
Properties
Potassium permanganate is a dark purple, crystalline solid (melting point 523 K) and is soluble in water. It is thermally unstable and decomposes as
⇒ \(2 \mathrm{KMnO}_4 \stackrel{\Delta}{\longrightarrow} \mathrm{K}_2 \mathrm{MnO}_4+\mathrm{MnO}_2+\mathrm{O}_2\)
It is a powerful oxidising agent in alkaline, neutral and acidic mediums. In an alkaline medium gets reduced to MnO2, in a neutral medium to manganate and in an acidic medium to manganese(2). The half-reactions of the reduction of permanganate to manganate, manganese dioxide and manganese(2) respectively may be represented as.
⇒ \(\mathrm{MnO}_4^{-}+\mathrm{e}^{-} \rightarrow \mathrm{MnO}_4^{2-} \quad\left(E^\theta=0.56 \mathrm{~V}\right)\)
⇒ \(\mathrm{MnO}_4^{-}+2 \mathrm{H}_2 \mathrm{O}+3 \mathrm{e}^{-} \rightarrow \mathrm{MnO}_2+4 \mathrm{OH}^{-}\left(E^{\ominus}=1.52 \mathrm{~V}\right)\)
⇒ \(\mathrm{MnO}_4^{-}+8 \mathrm{H}^{+}+5 \mathrm{e}^{-} \rightarrow \mathrm{Mn}^{2+}+4 \mathrm{H}_2 \mathrm{O} \quad\left(E^{\ominus}=1.52 \mathrm{~V}\right)\)
Thus the oxidising power of potassium permanganate is pH dependent. Although the course of a reaction is obvious from its redox potential, some reactions may seem to be thermodynamically allowed (for example oxidation of water at pH = 1) but actually do not occur as the rate of the reaction is too slow.
In an alkaline medium, potassium permanganate oxidises iodide to iodate, thiosulphate to sulphate and manganese) salts to manganese(2) oxide. The half-reactions for the oxidation processes are as follows.
⇒ \(\underset{\text { Iodide ion }}{\mathrm{I}^{-}}+6 \mathrm{OH}^{-} \rightarrow \underset{\text { Iodate ion }}{\mathrm{IO}_3^{-}}+3 \mathrm{H}_2 \mathrm{O}+6 \mathrm{e}^{-}\)
⇒ \(\underset{\text { Thiosulphate ion }}{\mathrm{S}_2 \mathrm{O}_3^{2-}}+10 \mathrm{OH}^{-} \rightarrow \underset{\text { Sulphate ion }}{2 \mathrm{SO}_4^{2-}}+5 \mathrm{H}_2 \mathrm{O}+8 \mathrm{e}^{-}\)
⇒ \(\mathrm{Mn}^{2+}+4 \mathrm{OH}^{-} \rightarrow \mathrm{MnO}_2+2 \mathrm{H}_2 \mathrm{O}+2 \mathrm{e}^{-}\)
The overall redox reaction can be written by adding the half-reaction of the reductant to the half-reaction for KMnO4, Le.,
⇒ \(\mathrm{MnO}_4^{-}+2 \mathrm{H}_2 \mathrm{O}+3 \mathrm{e}^{-} \rightarrow \mathrm{MnO}_2+4 \mathrm{OH}^{-}\)
After multiplying with suitable integers, the balanced overall reactions are obtained as follows.
⇒ \(2 \mathrm{MnO}_4^{-}+\mathrm{I}^{-}+\mathrm{H}_2 \mathrm{O} \rightarrow 2 \mathrm{MnO}_2+\mathrm{IO}_3^{-}+2 \mathrm{OH}^{-}\)
⇒ \(8 \mathrm{MnO}_4^{-}+3 \mathrm{~S}_2 \mathrm{O}_3^{2-}+\mathrm{H}_2 \mathrm{O} \rightarrow 8 \mathrm{MnO}_2+6 \mathrm{SO}_4^{2-}+2 \mathrm{OH}^{-}\)
⇒ \(2 \mathrm{MnO}_4^{-}+3 \mathrm{Mn}^{2+}+2 \mathrm{H}_2 \mathrm{O} \rightarrow 5 \mathrm{MnO}_2+4 \mathrm{H}^{+}\)
As already stated potassium permanganate gets reduced to manganese(2) in an acidic medium.
An acidified potassium permanganate solution oxidises oxalates to carbon dioxide, iodide to iodine, iron(2) salts to iron(3) salts, nitrites to nitrates, sulphites to sulphates and sulphides to sulphur.
The half-reactions of the same are as follows.
C2O4-2→2CO2+2e–
2I–→I2+2e–
Fe2+→Fe3++e–
NO2–+H2O→SO4-2+2H++2e–
SO32-+H2O→SO42-+2H++2e–
S2→S+2e–
By using techniques already known to you, you can easily write the complete ionic equations of the overall reactions. For example,
⇒ \(2 \mathrm{MnO}_4^{-}+5 \mathrm{C}_2 \mathrm{O}_4^{2-}+16 \mathrm{H}^* \rightarrow 2 \mathrm{Mn}^{2+}+10 \mathrm{CO}_2+8 \mathrm{H}_2 \mathrm{O}\)
Structure
In a permanganate ion, the manganese atom is tetrahedrally surrounded by four oxygen atoms. The structure of the permanganate ion is similar to that of the CIO4 or SO2 ions.
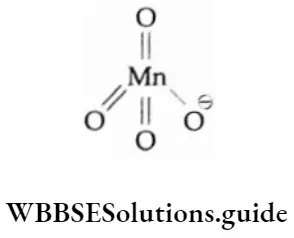
The double bonds arise due to the overlap of the p orbitals of oxygen with the d orbitals of manganese. The permanganate ion is diamagnetic in nature.
Potassium permanganate contains manganese(7), i.e., a d° electron configuration, and its crystals are an intense purple in colour.
The structure of the manganate ion, MnO42-, is somewhat similar to that of the permanganate ion. It has an unpaired electron and is paramagnetic in nature.
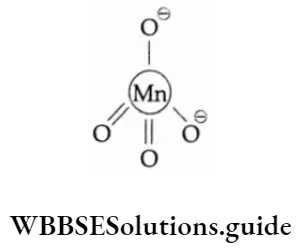
Uses
Potassium permanganate is mainly used as an oxidising agent in the laboratory and industry. It is employed as a volumetric agent in redox titrations. Potassium permanganate is also used as a disinfectant and a bleaching agent for cotton, silk and wool.
The bleaching action of potassium permanganate is due to its oxidising power. It is also used for the decolourisation of oil. Alkaline potassium permanganate is referred to as Baeyer’s reagent and is widely used as an oxidation organic synthesis. It is used to check unsaturation in organic compounds.
The f-block Elements
As already stated earlier in the chapter the f-block elements have partially filled f orbitals and they are also known as inner transition elements. The f-block elements are divided into two groups lanthanoids and actinoids.
The lanthanoids comprise lanthanum (La) and the fourteen elements that follow it in the series. The elements closely resemble lanthanum and hence the latter is included in any discussion of the lanthanoids which are generally collectively represented by the symbol Ln. The lanthanoids show very close similarity in properties with each other. Also, they exhibit only one stable oxidation state.
The Lanthanoids
In the series, lanthanum is the preceding d-block element with the outermost electronic configuration 5d 6s.In the remaining fourteen elements the electrons are filled up in the antepenultimate 4f orbital on moving across the period. The names, atomic numbers, symbols and electronic configurations of the element and the tripositive cation (most common oxidation state), as well as atomic and ionic radii, are given.
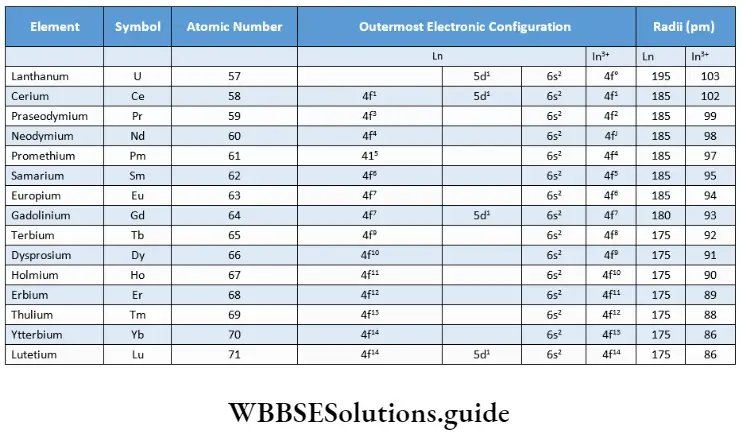
Note: The electronic configuration outside the xenon core is indicated.
We will now discuss the important general characteristics of the lanthanoids.
Electronic configuration
If you carefully study the outermost electronic configurations of lanthanoids, you will find certain irregularities. For instance, the electronic configuration of Pr is 4f3 6s2 and not 4f2 5d1 6s2 as expected.
The reason is that the energies of the 5d and 4f orbitals are very similar and therefore it is energetically favourable to move the single 5d electron to the 4f level in most of the elements. However, this does not happen in gadolinium (Gd), as the half-filled 4f orbital gives increased stability.
The electronic configurations of the trivalent ions (most stable oxidation state), however, are of the form 4fn (n =1 to 14) for all lanthanoids.
Atomic and ionic radii
There is a progressive decrease in atomic and ionic radii of elements from lanthanum to lutetium. This is referred to as the lanthanoid contraction. Usually, the atomic and ionic radii decrease on moving from left to right period. This is because of the incomplete shielding of the extranuclear charge.
In lanthanoids, an increase in nuclear charge is accompanied by a simultaneous increase in the number of 4f electrons. The 4f electrons shielders of nuclear charge. The shielding effect of electrons increases in the order f < d < p < s.
Consequences of lanthanoid contraction
- Due to the contraction in atomic size across the lanthanide series, the elements that follow in the 5d series are considerably smaller than expected. As a result, there is a close similarity in the atomic size of the elements in the 4d and the 5d series.
For example, the pairs of elements Zr-Hf, Nb-Ta and Mo-W have almost identical sizes. Thus Zr and Hf occur together in nature and are difficult to separate from each other. - Because of their similarity in size, the properties of the lanthanoids are very similar.
- As the size decreases from La3+ to Lu3+, the ionic character of the hydroxide, Ln(OH)3, decreases. In other words, the basicity decreases on moving across the series. So La(OH3 and Ce(OH)3 are the strongest bases.
Oxidation states
All lanthanoids exhibit a stable common oxidation state of +3. In addition, some elements show +2 and +4 oxidation states. These oxidation states are particularly exhibited when attaining their results in a noble-gas configuration, for example, Ce4+ (f0) or a half-filled f orbital, for example, Eu2+ (f7) or a completely-filled f orbital, for example, Yb2+ (f14 )
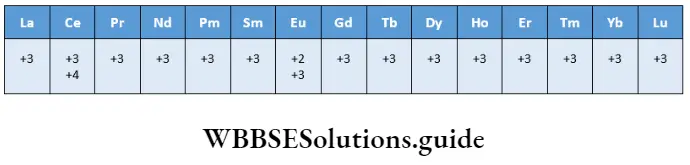
Note: The oxidation states shown in bold typeface are the most stable ones.
Ce(4) is a strong oxidising agent (Eθ for Ce4+/Ce3+ is + 1.74 V) and therefore it can thermodynamically oxidise water, but the reaction rate is very slow and Ce(4) is a good analytical reagent. Tb(4) too is an oxidant.
Praseodymium, dysprosium, neodymium and terbium form oxides of the type LnO2 where the metal shows a +4 oxidation state. (In these cases Ln4+ does not have an f0, f7 or f14 configuration.) Europium(2) has an f7 configuration.
However, it is a reducing agent and changes to the more stable +3 state. Ytterbium(II) has an f14 configuration and is a reductant. These examples highlight the fact that the most stable oxidation state for the lanthanoids is +3.
Ionisation enthalpies
Due to the similarity in size, the first and second ionisation enthalpies do not show a marked variation. The first ionisation enthalpies of the lanthanoids are roughly 600 kJ mol-1 while the second ionisation enthalpies are about 1200 kJ mol-1, which is comparable to that of calcium (the sizes of Ca+ and Ln+ are quite similar).
Variations are observed in the third ionisation enthalpy where it is seen that the values for lanthanum, gadolinium and lutetium are abnormally low. This is due to the extra stability associated with empty, half-filled or completely filled orbitals.
The outer electronic configurations of La, Gd and Lu are 5d1 6s2, 4f7 5d1 6s2 and 4f14 5dl 6s2 respectively. The bivalent cation easily loses the third electron as this results in a very stable configuration (that of La is 5d0, that of Ga3+is 4f7 and the configuration of Lu3+is 4f14)
Chemical reactivity
The lanthanoids are silvery white electropositive metals which tarnish in the air. The hardness increases with atomic number. Samariums are very hard and have a high melting point. The elements are good conductors of heat and electricity.
The metals are reactive, the earlier members of the series resemble calcium whereas the later members behave more like aluminium. They react with almost all elements including nitrogen and liberate hydrogen from acids. The metals form carbides when heated with carbon. They bum in halogens to form halides and form hydroxides and oxides.
Uses
Lanthanoids find little use in the pure state. They are mostly used in alloys. An important alloy is mischmetal which consists of lanthanoid metals (-95% lanthanoids) along with the other rare earths. It is alloyed with boron in lighter flints and is used in small quantities to increase the malleability of iron.
It is also used in magnesium alloys, which are employed in aircraft and bullets. Pyrophoric alloys are used in ignition devices and lighter flints. Mixed oxides of lanthanoids are employed as catalysts in petroleum cracking. Some oxides are used in television screens and fluorescent glasses.
The Actinoids
The actinoids comprise the fourteen elements that follow actinium, i.e., from thorium to lawrencium. Many of the members of this series are radioactive and the later members have short half-lives, which makes their study difficult. shows the atomic numbers, electronic configurations and atomic and ionic sizes of actinoids.
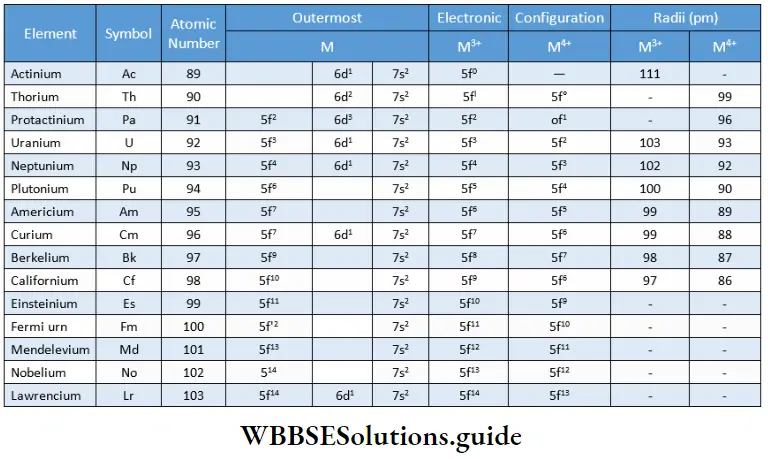
Electronic configuration
The outermost electronic configurations of the preceding elements Fr and Ra are 7s1 and 7s2 respectively. For the following element actinium (Ac), the 7s orbital is completely filled and the filling of the penultimate shell (d) begins so that its outer electronic configuration is 6d17s2.
Thus the actinoids have a filled 7s subshell and the 5f subshell is filled after thorium, i.e., from protactinium. The number of electrons in the 5f and 6d subshells varies. Certain irregularities in the configuration are observed due to the extra stability associated with the f0, f7 and f14 configurations as you have studied in the case of lanthanoids.
The 5f orbitals extend into space beyond the 6s and 6p orbitals and participate in bonding. This is in contrast to the lanthanoids where the 4f orbitals are totally shielded by the outer orbitals and therefore do not participate in bonding.
Oxidation states
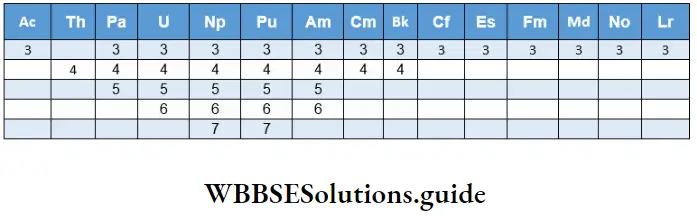
Since the 5f, 6d and 7s orbitals are comparable in energy and since electrons in the 5f orbital participate in bond formation, a wide range of oxidation states is displayed, particularly by the earlier elements in the series after thorium.
The +3 oxidation state is the most common and important for the later elements. The highest oxidation state is +7 for neptunium and plutonium after which the higher oxidation states become less stable. The M3+ and M4+ ions tend to hydrolyse.
Atomic and ionic sizes
As in the case of lanthanoids, there is a gradual decrease in the size of atoms and M3+ ions across the series. This is due to the poor shielding of the extra charge on the nucleus by 5f electrons.
This is referred to as actinoid contraction. A comparison of the ionic radii of trivalent ions of lanthanoids and actinoids shows that the ions are of comparable size; hence their properties are also somewhat similar.
Ionisation enthalpy
Actinoids have lower ionisation enthalpies than those of lanthanoids. The 5f electrons are effectively shielded from the nuclear charge and the outer electrons are less firmly held.
Physical and chemical reactivity
The actinoids are silvery metals, highly reactive in nature. They exist in different structural forms because of their irregular metallic radii. They react with hot water and tarnish in the air, forming an oxide coating.
They react readily with hydrochloric acid but turn passive by the action of concentrated nitric acid due to the formation of a protective oxide layer on the metal surface. These metals react with oxygen, hydrogen and halogens but not with sodium hydroxide.
Comparison of lanthanoids and actinoids
Some of the main similarities and differences between the lanthanoids and actinoids are listed as follows.
Similarities
- Both have partially filled f orbitals.
- They are electropositive metals.
- The main oxidation state displayed is +3.
- They exhibit magnetic properties and form coloured compounds.
Differences
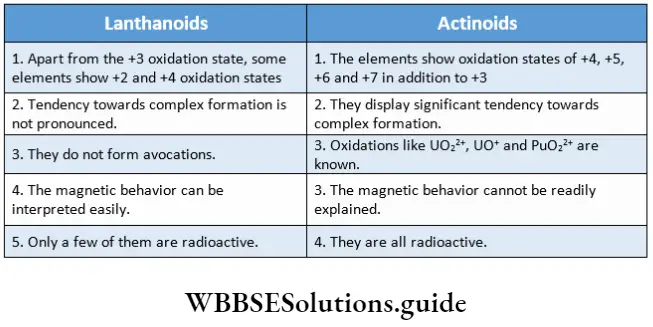
The d- and f-block Elements Multiple-Choice Questions
Question 1. The electronic configuration of Lu2+ is
- [Xe]4f14 Sd1 62
- [Xe]4f145d1
- [Xe]4f96s2
- [Xe]4f7 d1
Answer: 2. [Xe]4f145d1
Question 2. Paramagnetism arises due to
- Paired electrons
- A lone pair of electrons
- An unpaired electron
- None of the above
Answer: 3. An unpaired electron
Question 3. Misch metal is an alloy of
- Mn, Fe and C
- Mn, Cr and W
- Cu, Zn and Ni
- Lanthanoids and rare earths
Answer: 4. Lanthanoids and rare earth
Question 4. Green vitriol is
- FeSO4 .7H2 O
- Fe2 (SO4) 3
- CuSO4 -5H2 0
- CuSO4
Answer: 1. FeSO4 .7H2 O
Question 5. The reducing agent for iron oxide in the blast furnace is
- Carbon
- Limestone
- Carbon dioxide
- Carbon monoxide
Answer: 4. Carbon monoxide
Question 6. The silver salt widely used in photography is
- AgCl
- AgBr
- Agl
- AgF
Answer: 2. AgBr
Question 7. Galvanisation of iron is done by
- Cu plating
- Zn plating
- Ag plating
- Sn plating
Answer: 2. Zn plating
Question 8. Zn reacts with excess NaOH to give
- ZnH2
- ZnO
- Zn(OH)2
- Na2 [Zn(OH)4]
Answer: 4. Na2 [Zn(OH)4]
Question 9. Which among the following gives a colourless aqueous solution?
- Ni2+
- Fe2+
- Cu+
- Cu2+
Answer: 3. Cu+
Question 10. K2[HgI4] is used to detect
- NH4+
- NH2
- Na+
- NO3
Answer: 1. NH4+
Question 11. Which of the following is not coloured?
- Na2[CuCl4]
- Na2[CdCl4]
- K4[Fe(CN)6]
- K3[Fe(CN)6]
Answer: 2. Na2[CdCl4]
Question 12. In the 3D transition series, as the nuclear charge increases, the screening effect
- Increases
- Decreases
- First decreases and then increases
- Does not change
Answer: 1. Increases
Question 13. Which of these is not a lanthanoid?
- Lu
- Eu
- Mo
- Gd
Answer: 3. Mo
Question 14. Manganese exhibits the maximum oxidation state in
- K2 Mn04
- KMnO4
- Mn3O4
- MnO2
Answer: 2. KMnO4
Question 15. Chromium exhibits the maximum oxidation state in
- KCr2O7
- Cr2O3
- CrO
- Cr2(SO4)3
Answer: 1. KCr2O7
Question 16. Which of the following is coloured?
- ZnSO4
- Ag2SO4
- CuCl
- CuCl2
Answer: 4. CuCl2
Question 17. What is the highest oxidation state of Ti?
- +5
- +6
- +4
- +3
Answer: 3. +4
Question 18 Due to the lanthanoid contraction, which of the following pairs of elements have similar sizes?
- Zr and Y
- Zr and Hf
- Zr and Zn
- Zr and Nb
Answer: 2. Zr and Hf
Question 19. Maximum number of oxidation states is displayed by
- Mn(Z=25)
- Fe(Z=26)
- Cr(Z=24)
- Co(Z=27)
Answer: 1. Mn(Z=25)
Question 20. Which of the following dissolves in hot NaOH?
- Fe
- Zn
- Cu
- Ag
Answer: 2. Zn
Question 21. Which of these forms a colourless solution in aqueous medium?
- Cr3+
- Ti3+
- Sc3+
- V3-
Answer: 3. Sc3+
Question 22. Which statement is not correct?
- La(OH), is less basic than Lu(OH)3.
- The atomic radii of Zr and Hf are the same.
- The ionic radius decreases from La3+ to Lu3+.
- The +3 oxidation state is commonly displayed by lanthanoids.
Answer: 1. La(OH), is less basic than Lu(OH)3.

