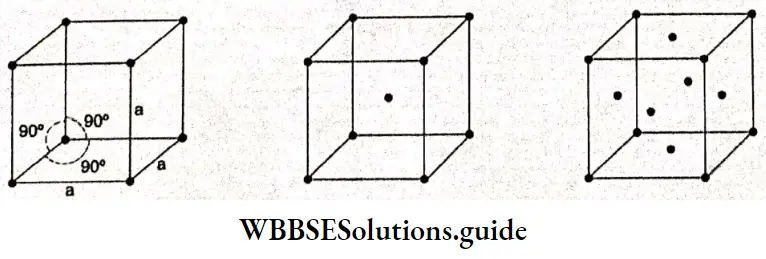
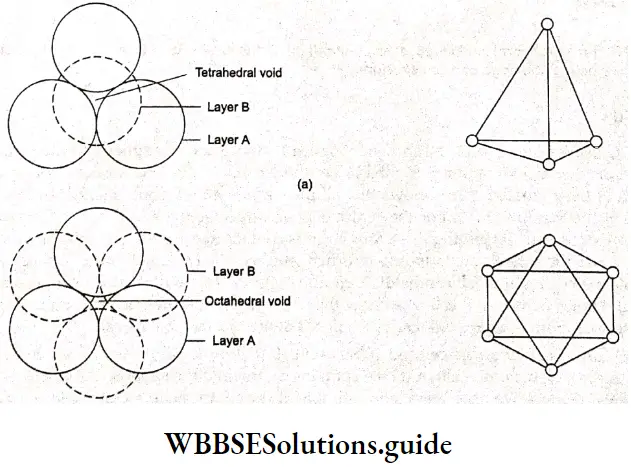
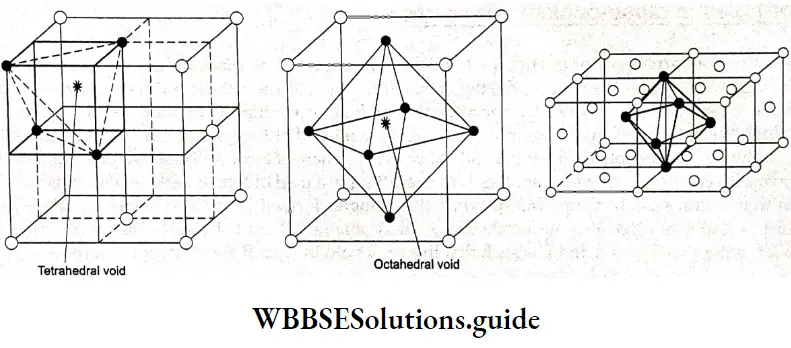
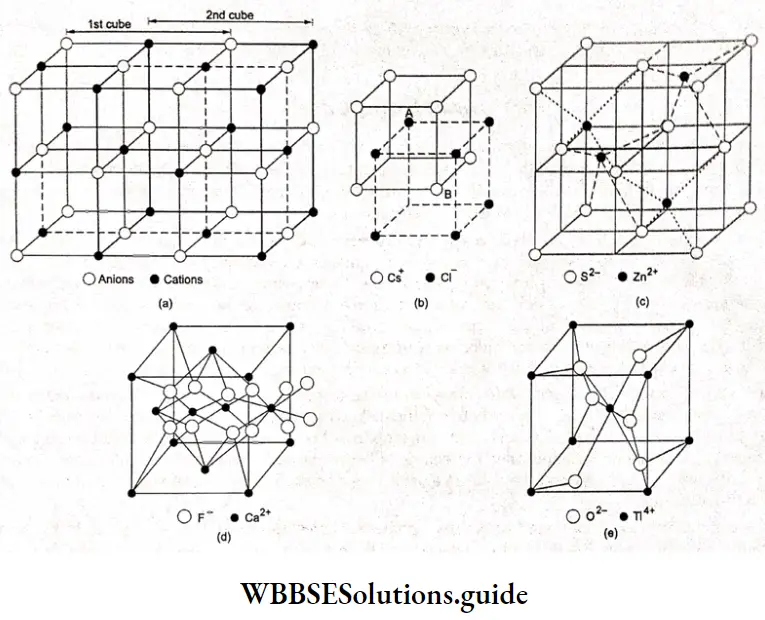
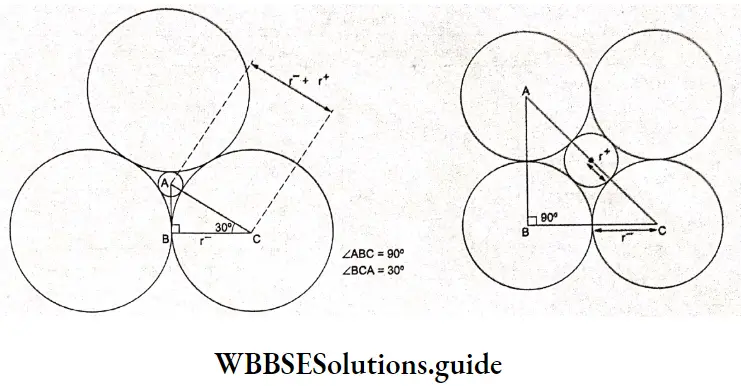
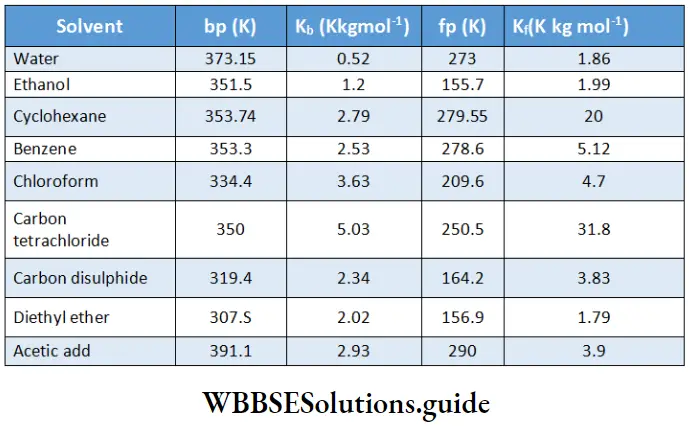
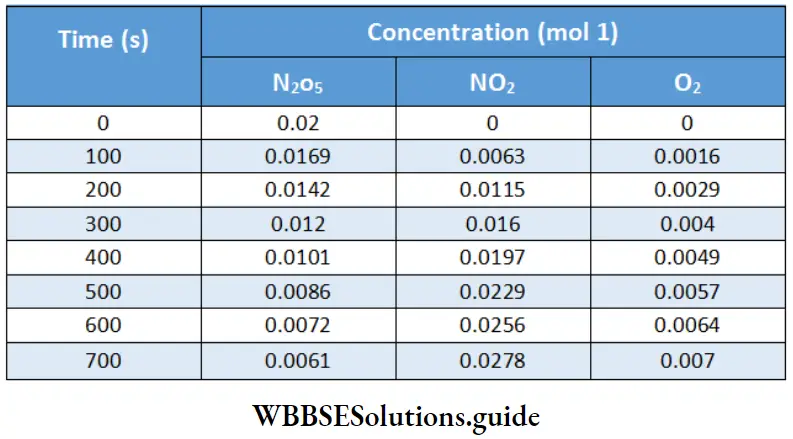
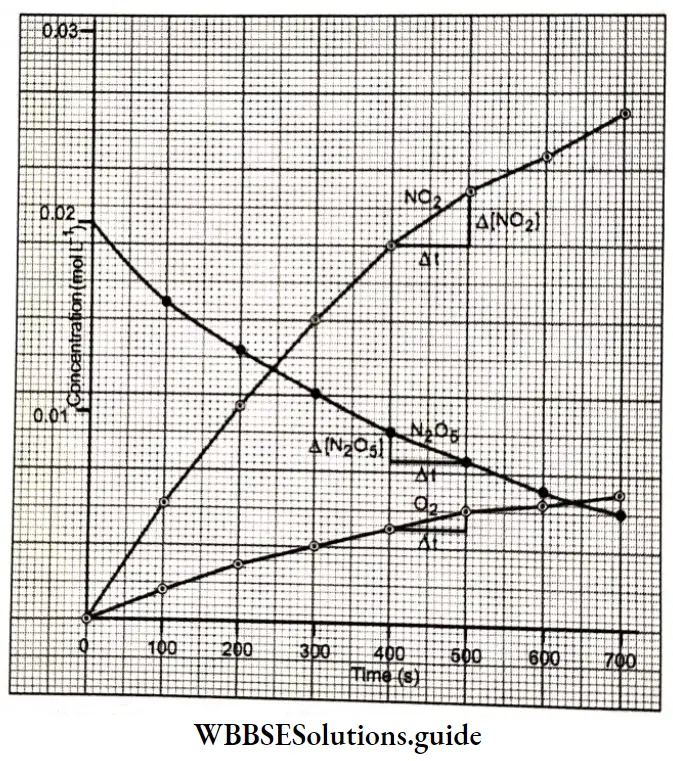
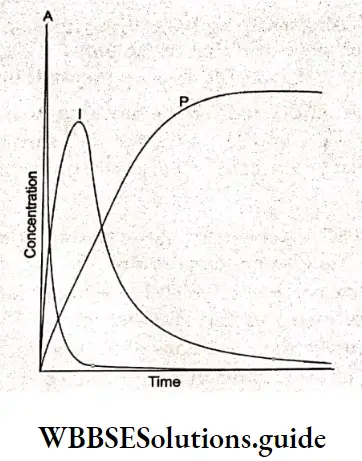
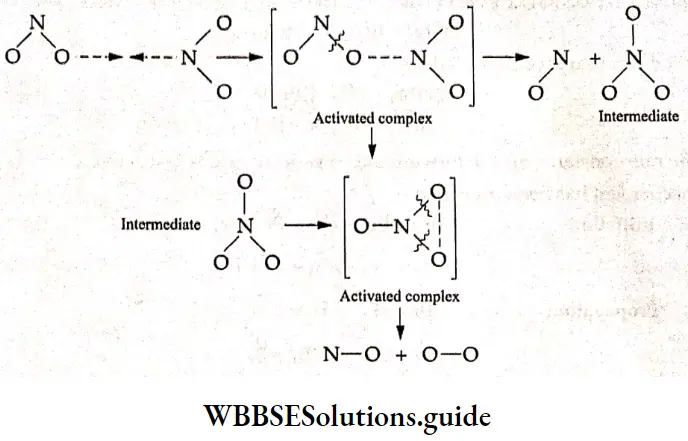
Electrochemistry
Electrochemistry:
Electrochemistry deals with the relationship between electrical energy and chemical energy and the interconversion of these two forms of energy. Chemical reactions can produce electrical energy. Also, certain nonspontaneous chemical reactions can be driven in the forward direction by applying an electric current.
Both these processes involve electrochemical reactions, i.e., chemical reactions using or generating electricity. The batteries we use in cars, toys, mobile phones, etc., are all examples of electrochemical cells. Methods like electroplating, employed to prevent corrosion, and electrorefining of metals involve electrochemical reactions.
Metallic and Electrolytic Conductance
Substances that allow the passage of electric current are called conductors. Metals are good conductors of electricity. Examples include copper, aluminum, and silver.
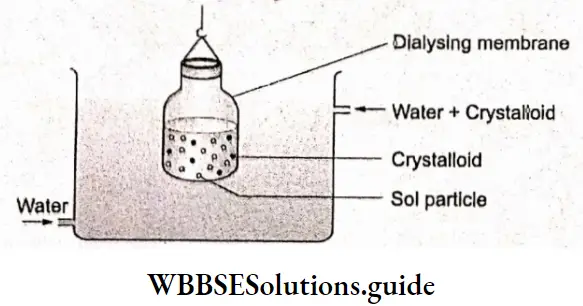
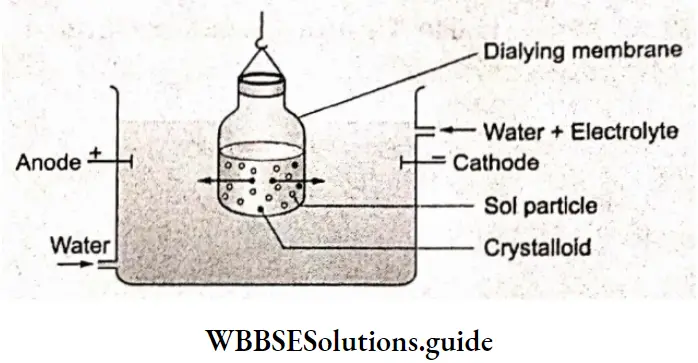
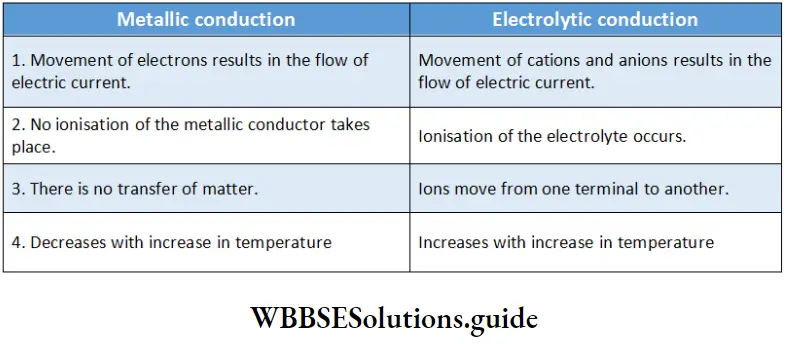
Apart from metals, there are other substances that allow the passage of electric current through them when they are in the molten state or in an aqueous solution. These substances are called electrolytes and the species responsible for the flow of current in electrolytes are the ions produced by the dissociation of these substances. This is in contrast to metallic conduction, in which the flow of current is due to the movement of free electrons.
The conduction of electricity by ions in solution is called ionic conduction. The conductivity of a solution depends upon various factors the
- Nature of the electrolyte
- The size of the ions in the solution,
- The nature of the solvent and its viscosity
- The temperature and concentration of the electrolyte.
On the other hand, metallic conduction occurs through electrons and is therefore also known as electronic conduction.
It depends on:
- The nature (density) and structure of the metal
- The number of valence electrons per atom
- Temperature.
Summarises the important characteristics of metallic and electrolytic conduction.
A comparison of metallic and electrolytic conduction:
Substances like sucrose, which do not conduct electricity either in their molten state or in their aqueous solutions, are called nonelectrolytes.
Conductance and Conductivity
Electrolytic conduction can be studied by applying a potential difference between two electrodes dipped in the solution of a sample. The ions of the sample which is an electrolyte move across the electrodes and result in the flow of electric current through the solution.
Under this condition, the electrolyte obeys Ohm’s law, according to which the potential difference between the electrodes, V, is equal to the product of the current I flowing through the solution and the resistance offered, R.
V=IR.
The reciprocal of resistance is called conductance, G, of the electrolytic solution.
\(G=\frac{1}{R}\)As resistance is expressed in Ω, conductance can be expressed in Ω. The SI unit of conductance is Siemens, S.
\(1 S=1 \Omega^{-1}\)Specific resistance and specific conductance
Consider a uniform bar of a conductor of length 1 cm and cross-sectional area A cm². Imagine that the cross-section is rectangular and that the bar is divided into cubes of side 1 cm.
The resistance of the bar is then equal to that of the I layers, in series with one another. Each layer is equivalent to A cubes, each cube of length 1 cm, whose resistances are in parallel. If p is the specific resistance (resistance of 1 cm cube) of the conductor, the resistance of one layer may be worked out from
\(\frac{1}{r}=\frac{1}{\rho}+\frac{1}{\rho}+\) ………. (A times)
Or \(\frac{1}{r}=A\left(\frac{1}{\rho}\right)\)
Or \(r=\frac{\rho}{A}\)
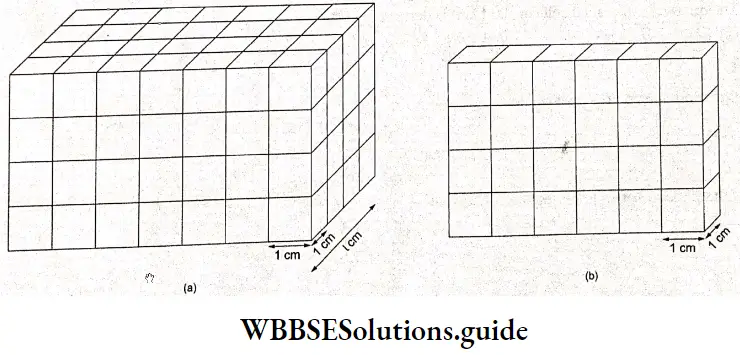
If R is the resistance of the whole bar, equivalent to the resistance of I layers, then
\(R=l r=l \cdot \frac{\rho}{A}=\rho \cdot \frac{l}{A} \Omega\)This equation is valid for all types of conductors including electrolytic solutions. In this equation, p is called resistivity (or specific resistance).
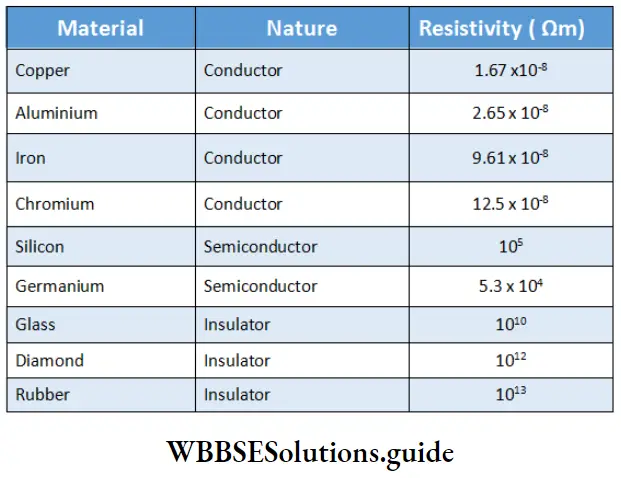
Substances can be classified as conductors, semiconductors, or insulators based on their resistivities. If I is expressed in cm and A in cm², the unit of p will be 2 cm. The SI unit is ohm m (22 m). The reciprocal of p is called specific conductance, or conductivity, denoted by K.
\(\kappa=\frac{1}{\rho}\)Resistivities of some materials:
Equation 3.2 can now be written as
\(R=\frac{1}{\kappa} \cdot \frac{l}{A}\)The quantity \(\frac{l}{A}\) is called cell constant and is denoted by G*.
\(G^*=\frac{l}{A}\)Equation 3.3 can now be written as
\(R=\frac{G^*}{\kappa}\)or, \(G^*=R \kappa\)
Taking the reciprocal of Equation 3.2, we get
\(\frac{1}{R}=\frac{1}{\rho} \cdot \frac{A}{l}\)or, \(G=\kappa \cdot \frac{A}{l}\)
From Equation 3.4 it may be seen that the conductance G of a solution decreases with 1 and increases with A.
The SI unit of K may be obtained as follows.
\(\mathrm{\kappa}=G \cdot \frac{l}{A}=\frac{1}{R} \cdot \frac{l}{A}=\frac{1}{\Omega} \cdot \frac{\mathrm{m}}{\mathrm{m}^2}=\Omega^{-1} \mathrm{~m}^{-1}=\mathrm{S} \mathrm{m}^{-1}\)Lists the conductivity values of some electrolytes:
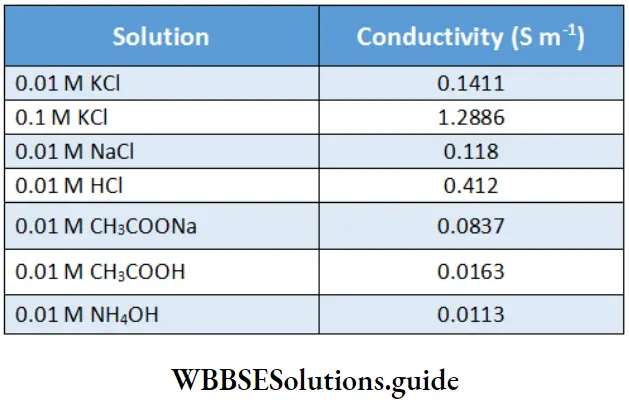
Conductivity may be defined as the conductance of a solution held between electrodes of a unit area of cross-section (1 cm2 or 1 m2) and unit distance apart (1 cm or 1 m). It may be noted that
\(1 \mathrm{~S} \mathrm{~cm}^{-1}=1 \frac{\mathrm{S}}{\mathrm{cm}}=1 \frac{\mathrm{S}}{10^{-2} \mathrm{~m}}=100 \mathrm{~S} \mathrm{~m}^{-1}\)Measurement Of The Conductivity Of Ionic Solutions
You must have studied in your physics lessons that the unknown resistance of a solid conductor can be measured using a Wheatstone bridge. However the resistance of an ionic solution cannot be measured by using a Wheatstone bridge because a solution cannot be connected to the bridge like a metallic wire.
Therefore, a specially designed conductivity cell is used to measure the electrical resistance of a solution of known concentration. Unlike in the Wheatstone bridge, where d.c. is used, in a conductivity cell, alternating current is used to minimize redox reactions.
Electrode reactions are prevented in an a.c. field since the electrodes change polarities very fast under the operating frequency. The reaction which occurs in the first half of the cycle is reversed in the second half.
The conductivity cell (in which the electrolyte is taken) consists of two platinum electrodes coated with platinum black. The electrodes have a cross-sectional area A and are a distance / apart.
In the conductivity cell, two known fixed resistances (Rx and R2) and a variable resistance R3 are arranged.
Tire current may flow along ABC and ADC as long as the potentials at B and D are different. The galvanometer will show a deflection. Once the two are balanced, no current flows and the galvanometer shows no deflection (null deflection). The resistance is adjusted to obtain null deflection on AB and at this point,
\(I_1 X=I_2 R_1 \text { and } I_1 R_3=I_2 R_2\)where X is the resistance of the electrolyte solution in the conductivity cell.
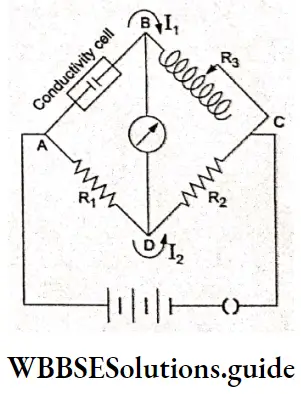
Hence \(\frac{R_3}{X}=\frac{R_2}{R_1}\)
\(X=R_3 \cdot \frac{R_1}{R_2}\)Instruments based on the above setup are called conductivity bridges. Nowadays instruments which directly give the conductivity value are also available.
They are called conductometers. There are many types of conductivity cells, varying in size and shape, but they can broadly be divided into two categories the solution is poured into the cell, which acts as a vessel, and another dip-type cell in which the cell is dipped inside a beaker containing the electrolyte solution.
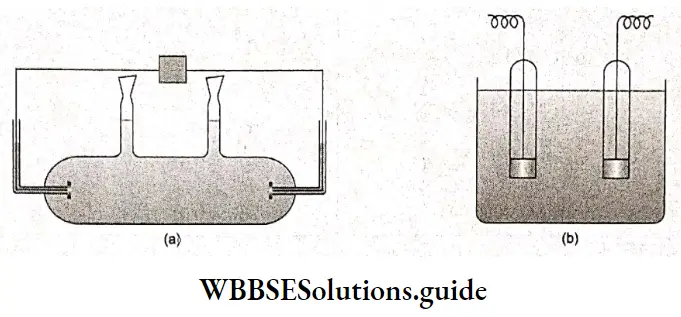
It is customary to determine the cell constant of the cell being used before measuring the conductance of the solution under study. For this purpose, the conductance of a solution of a known concentration of potassium chloride is measured.
For example, let us say the conductance of a 0.1 M KCl solution is observed to be 0.023 S. The specific conductance of this solution is noted from the literature and the cell constant is then calculated using the equation.
\(\frac{l}{A}=\frac{\kappa}{G}\)The cell constant for the cell in the above example, therefore, is found to be
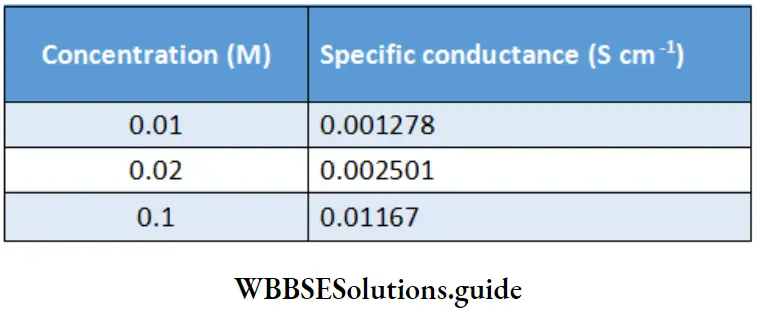
\(\frac{l}{A}=\frac{\kappa}{G}=\frac{0.01167}{0.023}=0.5 \mathrm{~cm}^{-1}\)Gives the conductivity values of some standard solutions of potassium chloride.
Specific conductance (conductivity) of KCl solutions at 20°C for various concentrations:
Molar conductivity
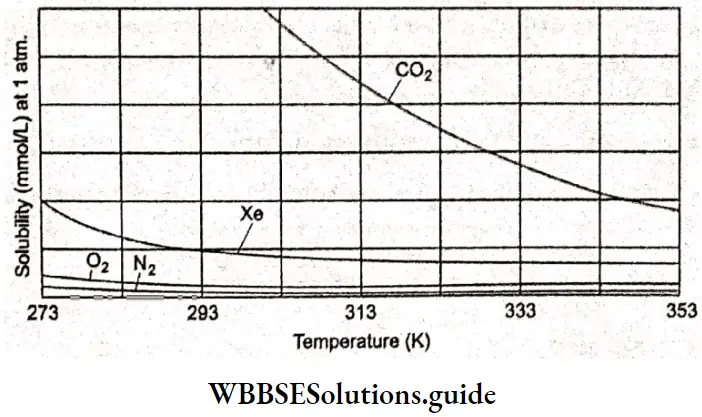
If the solution of one electrolyte is more concentrated than that of another, for a fixed volume of a solution held between the electrodes of a conductivity cell, the former will have more ions, and hence its conductivity may be higher.
To compare the conductances of different electrolytes, it was necessary to define another quantity, namely molar conductivity A. It is defined as the electrolytic conductivity к divided by concentration c.
Molar conductivity,
\(\Lambda_m=\frac{\kappa}{c}\)If the cell constant is known, the conductivity of any solution can be measured by filling the conductivity cell with the solution and measuring the resistance. Conductivity depends on the nature of the material.
The SI unit of A is S m² mol-1. If x is expressed in S m1, concentration should be expressed in mol m3. If c is expressed in mol L-1 (M), the formula becomes
\(\Lambda_m=\frac{\kappa\left(\mathrm{S} \mathrm{m}^{-1}\right)}{c\left(\mathrm{~mol} \mathrm{~L}^{-1}\right)}\) \(\frac{\kappa}{c} \cdot \frac{\left(\mathrm{S} \mathrm{m}^{-1}\right)(\mathrm{L})}{(\mathrm{mol})}\)\(\frac{\kappa\left(\mathrm{S} \mathrm{m}^{-1}\right)\left(\mathrm{dm}^3\right)}{c(\mathrm{~mol})}\) [… 1L = 1 dm3]
\(\Lambda_m=\frac{10^{-3} \kappa}{c} \mathrm{~S} \mathrm{~m}^2 \mathrm{~mol}^{-1}\)The CGS unit of A is S cm2 mol1. The two types of units are related to each other as
\(1 \mathrm{~S} \mathrm{~m}^2 \mathrm{~mol}^{-1}=10^4 \mathrm{~S} \mathrm{~cm}^2 \mathrm{~mol}^{-1}\)And \(1 \mathrm{~S} \mathrm{~cm}^2 \mathrm{~mol}^{-1}=10^{-4} \mathrm{~S} \mathrm{~m}^2 \mathrm{~mol}^{-1}\)
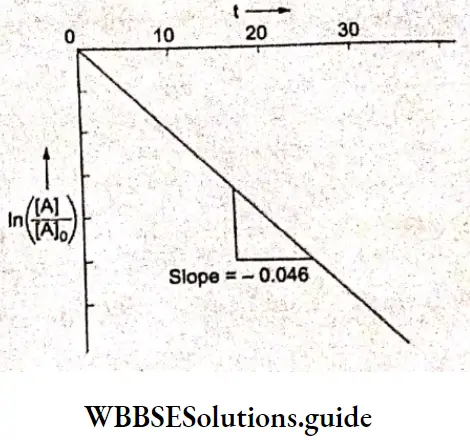
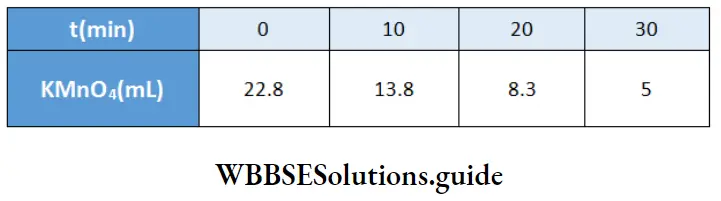
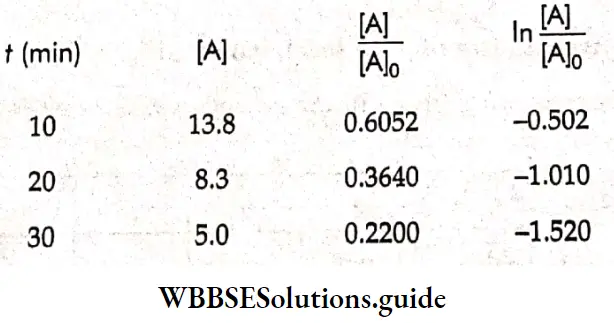
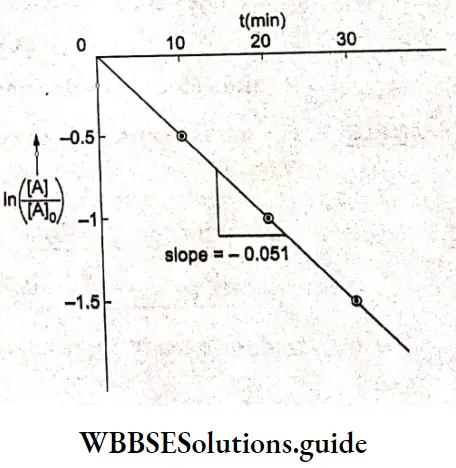
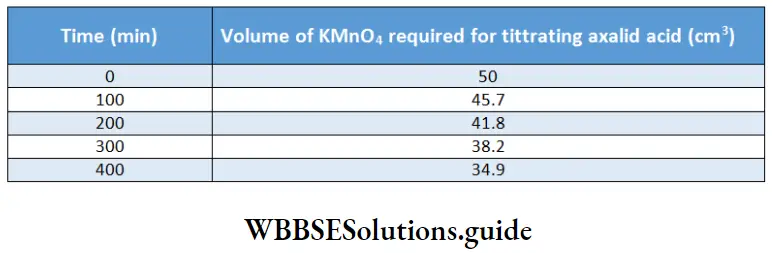
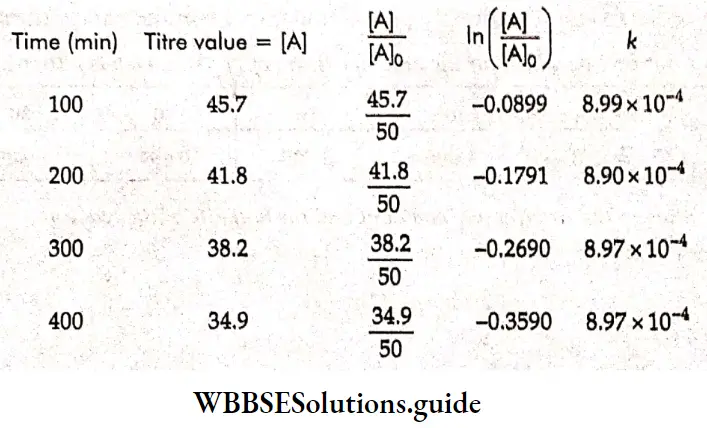
Example 1. The conductance of a solution of acid as read from a conductometer is 5×10 S. If the conductance of a 0.1 M KCl solution using the same cell and conductometer is found to be 1.07 x 10³ S, calculate the conductivity of the acid solution. The conductivity of a 0.1 M KCl solution is 0.001289 S cm¹.
Solution:
Given
The conductance of a solution of acid as read from a conductometer is 5×10 S. If the conductance of a 0.1 M KCl solution using the same cell and conductometer is found to be 1.07 x 10³ S
Whenever conductance has to be measured, first the conductance of a standard solution of KCl is determined and the cell constant is calculated. Then the conductance of the sample solution is determined and multiplied by the cell constant to obtain its conductivity.
\(\text { Cell constant } G^*=\frac{\text { conductivity } \mathrm{\kappa}}{\text { conductance } G}=\frac{0.001289}{1.07 \times 10^{-3}}=1.2 \mathrm{~cm}^{-1}\)Now conductivity of acid solution = K=5×10 -3 x 1.2=6×10 -3 S cm -1.
Example 2. The conductance of a 0.10 mol L-1 solution of NaCl was found to be 6.13×1032-1. Calculate its molar conductivity if the cell constant is 1.5 cm¹.
Solution:
Given
The conductance of a 0.10 mol L-1 solution of NaCl was found to be 6.13×1032-1.
For calculating molar conductivity, the conductivity has to be calculated.
K = \(G \cdot G^*=4 \times 0.005=0.02 \mathrm{~S} \mathrm{~m}^{-1}\)
\(9.195 \times 10^{-3} \Omega^{-1} \mathrm{~cm}^{-1} \text { or } \mathrm{Scm}^{-1}\)Molar conductivity,
\(\Lambda_m=\frac{\kappa \times 1000}{c(\text { in M })}=\frac{9.195 \times 10^{-3}}{0.1} \times 1000=0.0919 \times 10^3 \mathrm{~S} \mathrm{~cm}^2 \mathrm{~mol}^{-1}\)Example The conductance of a 0.1 M solution of an electrolyte of formula AX2 is 4@@@-1. Calculate the molar conductivity of this solution if the cell constant is 0.005 m -1.
Solution Conductivity K = \(G \cdot G^*=4 \times 0.005=0.02 \mathrm{~S} \mathrm{~m}^{-1}\)
Molar conductivity \(\)
But 10 dm = 1 m ⇒ 1dm = 0.1 m
∴ \(\Lambda_m=\frac{\kappa}{c}=\frac{0.02 \mathrm{~S} \mathrm{~m}^{-1}}{0.1 \mathrm{~mol} \mathrm{dm}^{-3}}\)
\(\frac{0.02 \times(0.1)^3}{0.1} \mathrm{~S} \mathrm{~m}^2 \mathrm{~mol}^{-1}=0.002 \mathrm{~S} \mathrm{~m}^2 \mathrm{~mol}^{-1}\)Example The conductance of 0.01 M HCl was found to be 3.296×103 using a cell in which the distance between the electrodes was 1 cm and the area of the cross-section of electrodes was 0.80 cm2. Calculate its molar conductivity.
Solution Cell constant \(\mathrm{G}^*=\frac{1}{0.80} \mathrm{~cm}^{-1}=1.25 \mathrm{~cm}^{-1}=1.25 \times \frac{1}{\mathrm{~cm}}=\frac{1.25}{10^{-2} \mathrm{~m}}\)
= 125 m-1
Conductivity K=G x G* = \(3.296 \times 10^{-3} \times 125 \mathrm{~S} \mathrm{~m}^{-1}=0.412 \mathrm{~S} \mathrm{~m}^{-1}\)
Molar conductivity = \(\frac{10^{-3} \times \kappa}{c\left(\mathrm{~mol} \mathrm{~L}^{-1}\right)}=0.0412 \mathrm{~S} \mathrm{~m}^2 \mathrm{~mol}^{-1}\)
Variation Of Conductivity And Molar Conductivity With Concentration
Conductivity always decreases with a decrease in concentration for all kinds of electrolytes (both strong and weak). This can be understood from the relation between conductance and conductivity.
When both the distance I and the area of cross-section a are unity, the conductance measured is the specific conductance or conductivity. We are basically measuring the conductance of one unit volume of solution to obtain the specific conductance.
When the concentration decreases, i.e., the solution becomes dilute, the same unit volume of solution contains fewer ions and hence the conductivity decreases. In contrast to conductivity, molar conductivity increases on dilution. We can relate the molar conductivity to the volume of the solution as follows.
\(\Lambda_m=\frac{\kappa}{c}\)If c is expressed in mol m-3
\(\Lambda_m=\kappa \times\left(\frac{1}{c}\right)=\kappa V\)where V is the volume of a solution containing 1 mol of the electrolyte (c moles contained in 1 m³; 1 mol contained in = \(\frac{1}{c} \mathrm{~m}^3\))
Am is directly related to the volume containing 1 mol of the substance. In a dilute solution, the volume containing 1 mol of the electrolyte will be larger and hence from Equation 3.6, A will be larger. As the concentration increases, the molar conductivity decreases.
We can therefore define molar conductivity as the conductance of a volume of a solution containing 1 mol of a dissolved substance when placed between two parallel electrodes which are a unit distance apart and are large enough to contain between them the whole solution.
Strong And Weak Electrolytes
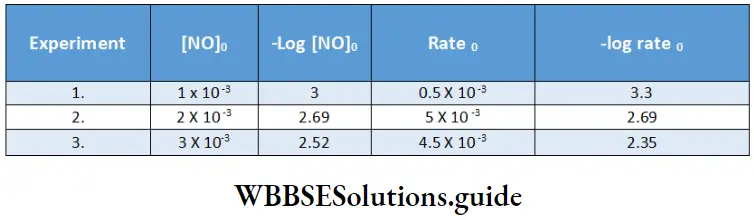
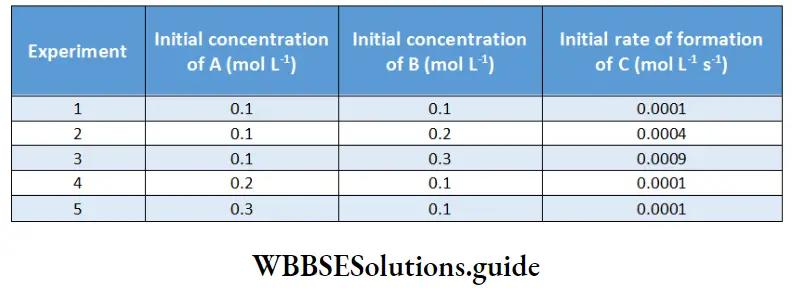
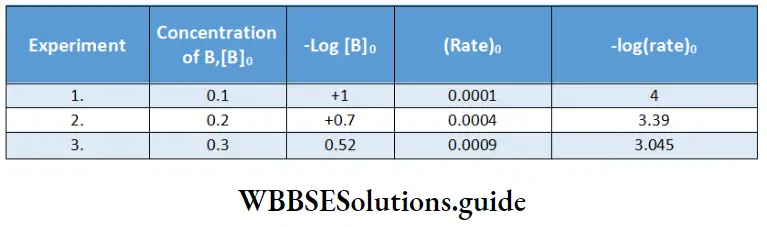
Between 1860 and 1880, Friedrich Kohlrausch and his coworkers performed a series of measurements of molar conductivities of aqueous solutions with varying concentrations of electrolytes. The molar conductivity was found to vary with the concentration of the electrolyte.
This variation is due to the fact that the number of ions per unit volume of the solution is not proportional to the molar concentration of the electrolyte. For example, in case of a weak acid, doubling the concentration of the acid does not double the number of ions. In addition, since ions interact with each other, the conductivity of a solution is not proportional to the number of ions present.
Based on the dependence of molar conductivity on concentration, electrolytes are divided into two classes-strong and weak. The characteristic of a strong electrolyte is that its molar conductivity decreases only slightly as its concentration is increased.
On the other hand, the molar conductivity of a weak electrolyte is appreciable at concentrations close to zero but falls sharply as the concentration increases. The classification of electrolytes also depends on the solvent employed. For example, lithium chloride is a strong electrolyte in water but a weak electrolyte in propanone.
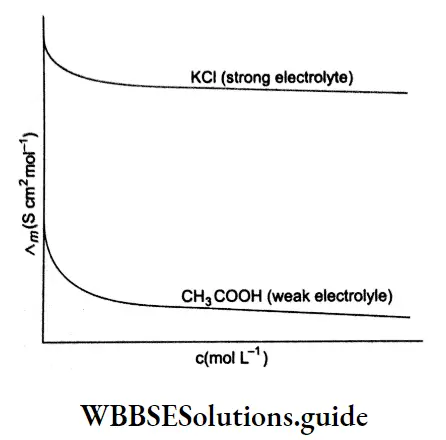
Strong Electrolytes
Strong electrolytes are completely ionized in solution. Examples include ionic solids such as NaCl and KCl, and strong acids. The number of ions in a solution of a strong electrolyte is proportional to the concentration of the electrolyte added.
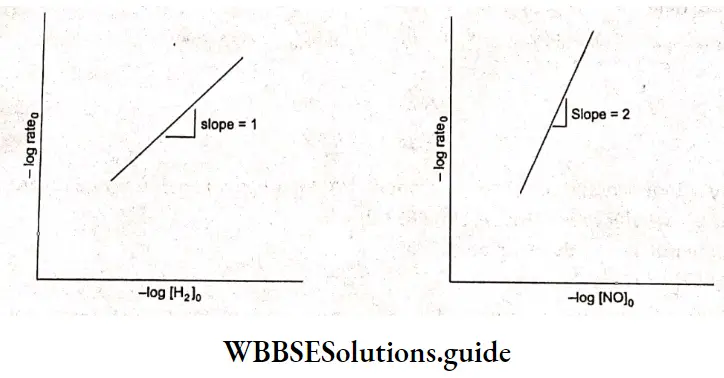
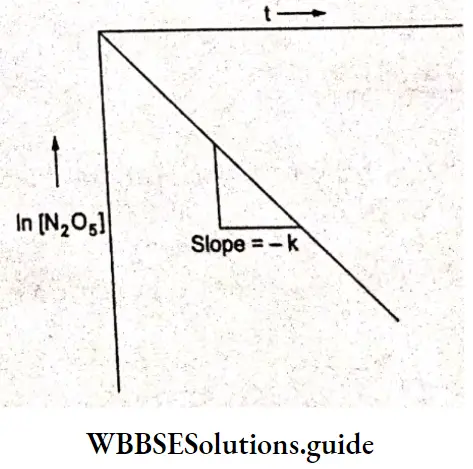
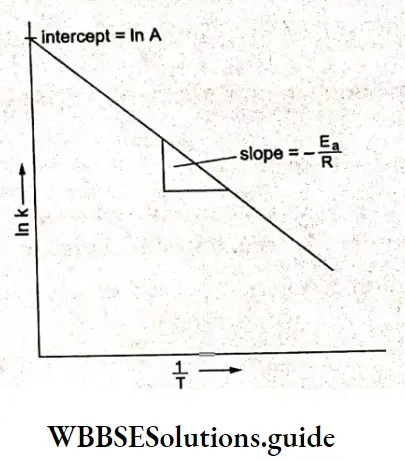
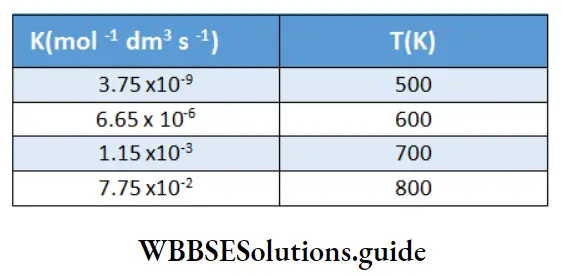
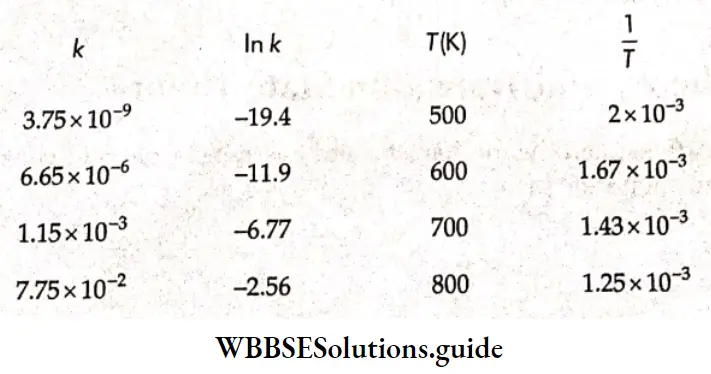
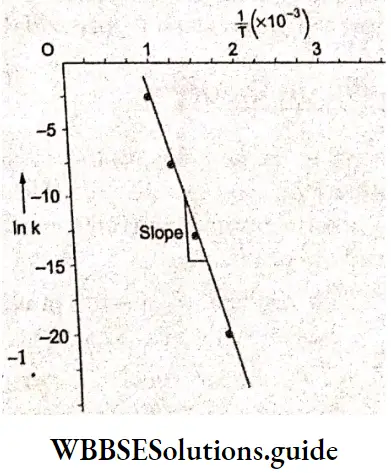
Kohlrausch, from a series of experiments, showed that at low concentrations the molar conductivities of strong electrolytes vary linearly with the square roots of their concentrations.
\(\Lambda_m=\Lambda_m^0-A \sqrt{c}\)This is called Kohlrauseh’s law. The constant \(\Lambda_m^0\), is the limiting molar conductivity, the molar conductivity in the limit of zero concentration. This means that it is the molar conductivity of the solution when its concentration approaches zero. In other words, it refers to a solution that is so dilute that there are very few ions and they are far apart and do not interact with one another.
\(\Lambda=\Lambda_m^0 \text { as } c \rightarrow 0\)Factor A is a constant and depends more on the stoichiometry of the electrolyte (i.e., whether it is MA, M2A, etc.) than on its specific identity. For example, NaCl and KCl are a particular type of electrolytes, i.e., 1-1, having the same value for A. Similarly CaCl2 and MgSO4 are respectively of types 2-1 and 2-2 respectively.
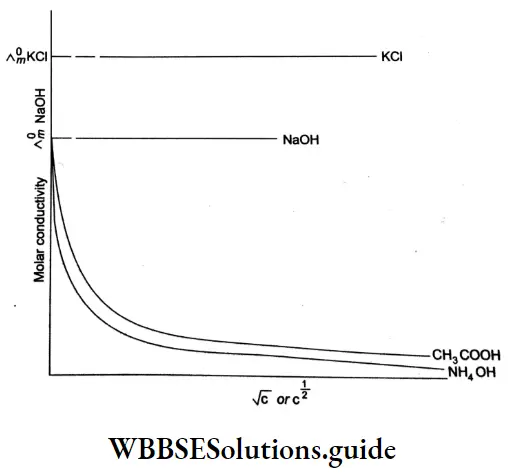
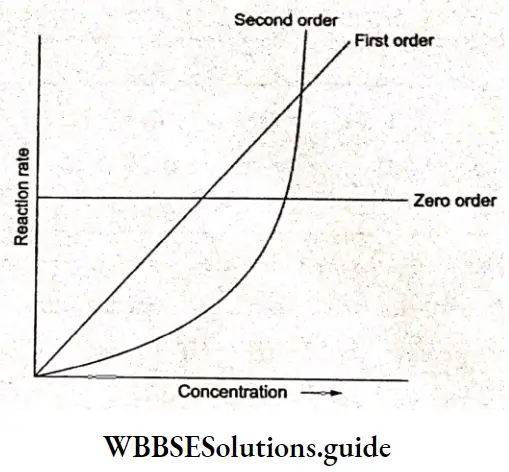
The value of \(\Lambda_m^0\), can be obtained by plotting \(\Lambda_m \operatorname{vs} \sqrt{c}\) and then extrapolating the graph to √c = 0. It can be seen that the plot of \(\Lambda_m \operatorname{vs} \sqrt{c}\) is nearly linear for KCl, a strong electrolyte, but \(\Lambda_m\) decreases slightly as the concentration increases.
The number of ions per unit volume of the solution increases as the concentration increases and an increase in Am is expected. However, there is a considerable change in interionic interaction (between K+ and Cl–) as the concentration changes. The attraction between the ions increases with an increase in concentration.
The ability of the ions to move independently decreases, as a result of which the conductivity decreases (but only slightly) as the concentration increases. As the solution is diluted, the concentration decreases, the ions are far apart, the attraction decreases, and the conductivity increases.
When the concentration becomes very low, the interionic interactions are the least and the molar conductivity approaches the maximum limiting value A°m, a constant. In other words, the limiting molar conductivity is the molar conductivity in the limit of such low concentration that ions are no longer able to interact with one another.
The tin value is characteristic of every strong electrolyte. It is also called the molar conductivity at infinite dilution. The ions are very well separated at infinite dilution or in the limit of zero concentration and therefore behave independently of one another.
Kohlrausch gave the law of independent migration of ions. The law states that the limiting molar conductivity of an electrolyte can be expressed as the sum of the individual contributions of the anion and cation of the electrolyte.
If the limiting molar conductivity of cations is denoted by X°+ and that of anions by λ°_, then according to Kohlrausch’s law of independent migration of ions,
\(\Lambda_m^0=v_{+} \lambda_{+}^0+v_{-} \lambda_{-}^0\)where v+ and v_ are tire numbers of cations and anions respectively per formula unit of the electrolyte. For example, for KC1 and \(v_{+}=v_{-}=1 ; \text { for } \mathrm{MgCl}_2, v_{+}=1, v_{-}=2\)
Weak Electrolytes
Weak electrolytes are not fully ionized in the solution. They include weak Bronsted acids and bases such as CH3COOH and NH3. The following equilibrium exists in solutions of weak electrolytes.
\(\mathrm{HA}(\mathrm{aq})+\mathrm{H}_2 \mathrm{O}(\mathrm{l}) \rightleftharpoons \mathrm{H}_3 \mathrm{O}^{+}(\mathrm{aq})+\mathrm{A}^{-}(\mathrm{aq})\)At low concentrations, the equilibrium shifts towards the right, thus increasing the number of ions in solution due to which the conductivity increases. Therefore, it is obvious that the conductivity depends on the degree of ionization, a, of the weak electrolyte. Therefore, if the molar concentration of the acid HA is c then the concentrations of HA, H2O+, and A- at equilibrium will be
\([\mathrm{HA}]=(1-\alpha) c,\left[\mathrm{H}_3 \mathrm{O}^{+}\right]=\alpha c \text { and }\left[\mathrm{A}^{-}\right]=\alpha c \text {. }\)The acidity constant or the acid dissociation constant is given by
\(k_a=\frac{\left[\mathrm{H}_3 \mathrm{O}^{+}\right]\left[\mathrm{A}^{-}\right]}{[\mathrm{HA}]}\)or, \(k_a=\frac{\alpha^2 c}{1-\alpha}\)
At infinite dilution (c→0), the electrolyte is fully ionized and the molar conductivity is A°nr According to Arrhenius, at infinite dilution, only a fraction a is actually present as ions in the solution, and the measured molar conductivity is given by
\(\Lambda_m=\alpha \Lambda_m^0\)or, \(\alpha=\frac{\Lambda_m}{\Lambda_m^0}\)
Substituting this value of an in Equation (3.8), we get
\(k_a=\frac{\left(\frac{\Lambda_m}{\Lambda_m^0}\right)^2 c}{1-\left(\frac{\Lambda_m}{\Lambda_m^0}\right)}=\frac{c \Lambda_m^2}{\Lambda_m^0\left(\Lambda_m^0-\Lambda_m\right)}\)Using Kohlrausch’s law of independent migration of ions \(\ Lambda_m^0te] for any electrolyte can be calculated from the X° of individual ions (Equation 3.7). Both the degree of ionization and dissociation constant for a weak electrolyte can be determined if [latex]\Lambda_m \text { and } \Lambda_m^0\) are known at a given concentration c.
Example Calculate the molar conductivity at infinite dilution (\(\Lambda_m^0\)) of acetic acid given that \(\Lambda_m^0\) of HC1, NaCl, and CH3COONa are 426,126 and 91 Ω-1 cm² mol-1respectively.
Solution \(\Lambda_{m, \mathrm{HAC}}^0=\Lambda_{m, \mathrm{NaAc}}^0+\Lambda_{m, \mathrm{HCl}}^0-\Lambda_{m, \mathrm{NaCl}}^0\left(\text { Ac stands for acetate, } \mathrm{CH}_3 \mathrm{COO}^{-}\right)\)
∴ \(\Lambda_{m, \mathrm{HAc}}^0=91+426-126=391 \mathrm{~S} \mathrm{~cm}^2 \mathrm{~mol}^{-1}\)
If the units are to be converted to S m² mol-1, since 1 cm = 10-2 m, 1 cm2 = 10-2 x 10-2 m², multiply by 10-4 m²
∴ \(\Lambda_{m, \mathrm{HAc}}^0=391 \times 10^{-4} \mathrm{~S} \mathrm{~m}^2 \mathrm{~mol}^{-1}\)
= 0.0391 Sm² mol-1
Example If the molar conductivity of 0.001 M acetic acid is 0.00492 S m² mol-1, and its\(\Lambda_m^0\) is 0.03907 S m² mol4, calculate the degree of dissociation, &&&. Also, find the equilibrium constant for the dissociation of this acid.
Solution \(\alpha=\frac{\Lambda_m}{\Lambda_m^0}=\frac{0.00492}{0.03907}=0.1259\)
\(\mathrm{HAc} \rightleftharpoons \mathrm{H}^{+}+\mathrm{Ac}^{-}\) \(k=\frac{\left[\mathrm{H}^{+}\right]\left[\mathrm{Ac}^{-}\right]}{[\mathrm{HAc}]}=\frac{(c \alpha)^2}{c(1-\alpha)}=\frac{c \alpha^2}{1-\alpha}\)Thus the dissociation constant of acetic acid (HAc) is
\(k=\frac{0.001 \times(0.1259)^2}{1-0.13}=1.94 \times 10^{-5}\)Electrochemical Cells
An electrochemical cell is a setup in which a spontaneous chemical reaction occurs to produce electrical energy or, a nonspontaneous chemical reaction is carried out by using an external source of are called galvanic cells or voltaic cells, and the latter, electrolytic cells All electrochem electrodes dipping into either one or two electrolyte solutions.
Current can pass. It may be made of a metal such as copper, silver zinc, etc. or electric Electrodes come in a variety of shapes-they may be wireSPplates or rodsd.
AnddectrolyteTs In The electrode and its surrounding electrolyte solution are contained in electrodes may or may not share the same compartment.
Compartment The two are made of zinc and copper. These electrodes are dipped in zinc sulfate and copper sulfate The electrodes are respectively, taken in different compartments. To complete the electrical circuit the solution (electrolytes) salt bridge. The following redox reaction occurs in the cell.
\(\mathrm{Zn}(\mathrm{s})+\mathrm{Cu}^{2+}(\mathrm{aq}) \rightarrow \mathrm{Zn}^{2+}(\mathrm{aq})+\mathrm{Cu}(\mathrm{s})\)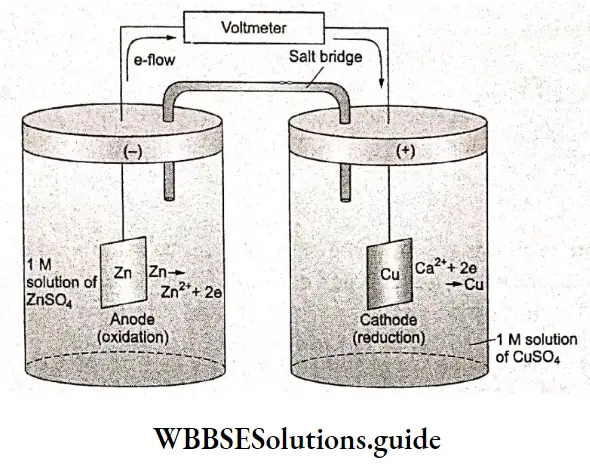
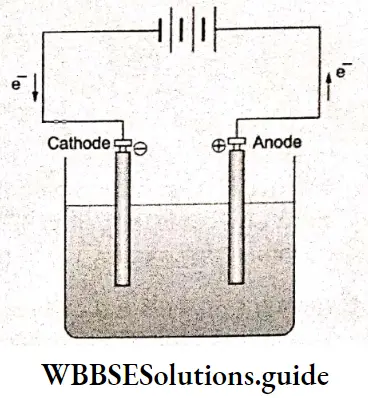
The chemical energy of this reaction is converted to electrical energy, which can be used to light a bulb, rim a motor, and so on.
An electrolytic cell. A battery supplies electrical energy to carry out a nonspontaneous reaction.
Cell diagram
Any electrochemical cell can be represented by a cell diagram that shows the oxidized as well as the reduced forms of the electroactive substance and any other species that may be involved in the chemical reaction.
The electrodes, denoted by their atomic symbols, are indicated at the two ends of the diagram. Any insoluble substances or gases are denoted inside, next only to the electrodes.
The soluble species are represented in the middle of the diagram. The states of aggregation (solid, liquid, or gas) are also specified within brackets. In an abbreviated diagram, some of the above information is ignored and only the main components are given.
In a cell diagram, a phase boundary is indicated by a solid vertical bar; a junction between two miscible liquid phases is represented by a single dashed vertical bar.
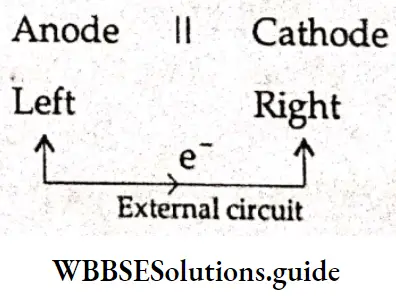
A double dashed vertical bar indicates a junction between two miscible liquid phases at which the junction potential has been eliminated using a salt bridge. Commas separate different soluble species in the same phase. It is assumed that the right-hand electrode is the cathode and the left-hand electrode is the anode.
Some examples of cell diagrams are given below.
1. \(\mathrm{Zn}(\mathrm{s})\left|\mathrm{Zn}^{2+}(a=0.35): \mathrm{Cu}^{2+}(a=0.49)\right| \mathrm{Cu}(\mathrm{s})\) (complete diagram)(a denotes the activity of the ions)
\(\mathrm{Zn}\left|\mathrm{Zn}^{2+}: \mathrm{Cu}^{2+}\right| \mathrm{Cu} \text { (abbreviated diagram) }\)This cell is called the Daniell cell.
2. \(\mathrm{Pt}\left|\mathrm{H}_2(\mathrm{~g})\right| \mathrm{HCl}(m)|\mathrm{AgCl}(S)| \mathrm{Ag}(\mathrm{s}) \mid \mathrm{Pt}\)
3. \(\mathrm{Pt}\left|\mathrm{H}_2(\mathrm{~g}), \mathrm{H}^{+}: \mathrm{Fe}^{3+}, \mathrm{Fe}^{2+}\right| \mathrm{Pt}\)
4. \(\mathrm{Zn}\left|\mathrm{Zn}^{2+}\left(c_1\right) \vdots \mathrm{Zn}^{2+}\left(c_2\right)\right| \mathrm{Zn}\)
(m denotes molality, c1 and c2 denotes the cons=centrations.)
Galvanic (Voltaic) Cell
A galvanic cell is an electrochemical cell that produces electricity from the spontaneous chemical reactions occurring inside it. Consider a cell made up of a zinc rod dipped in ZnSO4 solution and a copper rod dipped in
CuSO4 solution. This is a Daniell cell—a type of galvanic cell. To avoid direct mixing of the electrolyte solutions, these are taken in separate compartments, and connection between them is established through a salt bridge.
A salt bridge is made of a glass tube containing an inert electrolyte solution (whose ions do not react with the ions of the main electrolytes or the electrodes) such as KCl, KNOa, or NH4NO3, kept in agar-agar, a geL The two portions of the cell are called half-cells or redox couples.
In one half-cell, the metal from an electrode dissolves in the solution leaving behind electrons, making the electrode negatively charged and simultaneously, in the other half-cell, the metal ions from the solution deposit on the other metal electrode, making this electrode positively charged. The actual reactions occurring at the two electrodes (half-cell reactions) are as follows.
Zn electrode (anode) Oxidation occurs at this electrode.
\(\mathrm{Zn}(\mathrm{s}) \rightarrow \mathrm{Zn}^{2+}(\mathrm{aq})+2 \mathrm{e}^{-}\)Cu electrode (cathode) Reduction occurs at this electrode.
\(\mathrm{Cu}^{2+}(\mathrm{aq})+2 \mathrm{e}^{-} \rightarrow \mathrm{Cu}(\mathrm{s})\)The overall reaction is obtained by adding the above two equations.
\(\mathrm{Zn}(\mathrm{s})+\mathrm{Cu}^{2+}(\mathrm{aq}) \rightarrow \mathrm{Zn}^{2+}(\mathrm{aq})+\mathrm{Cu}(\mathrm{s})\)We can see that Zn undergoes oxidation while the Cu2+ ions undergo reduction. Thus the Zn electrode is negatively charged and the copper electrode is positively charged. The electrode which is negatively charged is called the anode and that which is positively charged is called the cathode. In the solution, the cations Zn2- and Cu2+ move towards the cathode while the anions SO4+ move towards the anode.
Since the two electrodes are oppositely charged, a potential difference occurs between them and electrons flow from the anode to the cathode. The direction of current is opposite to that of electron flow.
The conventional method is to write the reduction reactions for both electrodes (even though in reality oxidation may be occurring at one electrode) and obtain the overall reaction by subtracting the reduction reaction at the anode (left-hand electrode) from that occurring at the cathode (right-hand electrode). The half-reactions for the reduction of copper by zinc are as follows.
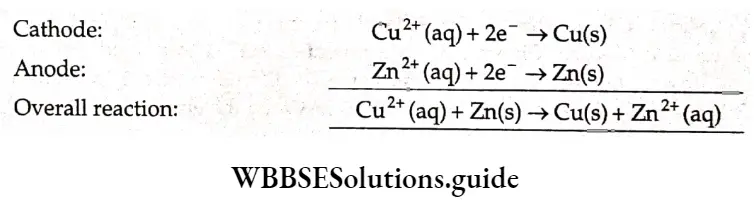
If the two solutions are not separated from each other, the Cu2+ ions react directly with the zinc bar.
\(\mathrm{Cu}^{2+}(\mathrm{aq})+\mathrm{Zn}(\mathrm{s}) \rightarrow \mathrm{Cu}(\mathrm{s})+\mathrm{Zn}^{2+}(\mathrm{aq})\)In such a situation, no useful electrical work is obtained.
The cell potential of a galvanic cell:
A galvanic cell converts the chemical energy liberated during a chemical reaction to electrical energy. It produces a driving force through the chemical reaction that pushes electrons through an external circuit. The work done by a given transfer of electrons depends on the cell potential.
The cell potential or emf of the cell is the potential difference between the two electrodes. It may also be called cell voltage. The unit of cell potential is the volt (V).
Under balanced reversible conditions, a cell reaction can proceed in either direction. Here eversibility refers to the change in direction of the movement of ions by reversing the polarity of the electrodes. In a cell diagram, the cell reaction is written in such a manner that electrons are shown to be accepted from the external circuit by the electrode on the right and given up by the electrode on the left.
Consider the following example In which ft cell In represents an
\(\mathrm{Pt}\left|\mathrm{H}_2(1 \mathrm{bar}) \& \mathrm{HCl}(1 \mathrm{M})\right| \mathrm{AgCl} \mid \mathrm{Ag}\)The reactions that occur in the electrodes of the cell are as follows,
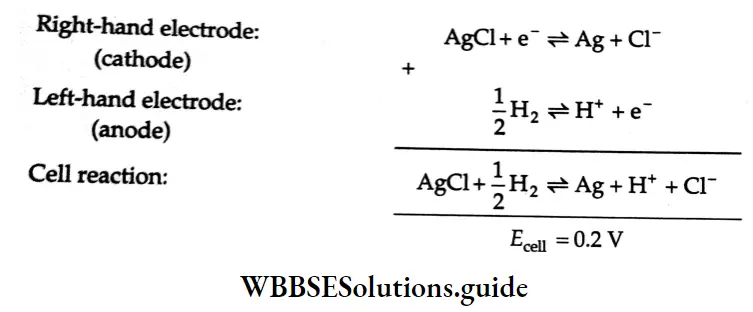
Conversely, if the above cell diagram is shown as ns
\(\left.\mathrm{Ag}|\mathrm{AgCl}| \mathrm{HCl}(1 \mathrm{M}) \mid \mathrm{H}_2 \text { (1 bar }\right) \mid \mathrm{Pt}\)then the reactions that occur at the two electrodes get reversed,
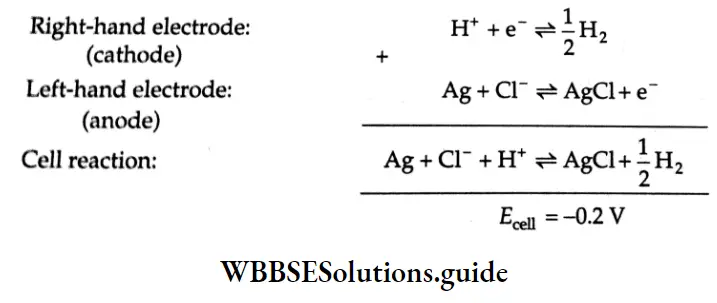
As you can see the emf of the reaction represented by Equation 3,9 Is positive and hence, by convention, the reaction will proceed from left to right. However, the emf of the reaction shown in Equation 3,10 is negative, indicating that the reverse of this reaction is spontaneous,
Measurement of the cell potential of a galvanic cell and that of a Daniell cell To measure the cell potential of a galvanic ceil, a potentiometer is placed in the circuit. In a potentiometer, a steady current from a battery flows through a resistor, A sliding contact is used to vary the potential difference across the slide wire connected to an electrochemical cell, If the applied potential difference (V) is less than the emf of the galvanic cell (Ecell) the cell discharges electricity spontaneously. When the two are exactly the same (V = Ea4l), then no current flows through the cell and this is the equilibrium potential difference (emf or Ece]|) we are interested in.
In practice, the two substances (electrodes and electrolytes) forming the cell are kept in their standard states (all the solutes are at 1 M and the gases are at 1 atm).
The cell potential measured under the standard state conditions is called the standard cell potential, Efcn. The superscript is used to indicate that all reactants and products are in their standard states.
When 1 M solutions of ZnSC)4 and CuSO4 are used for the redox reaction in the Daniell cell, the is found to be 1.1 V at 25°C. A galvanic cell has a positive emf if the cell reaction is spontaneous, i.e„ electrons are accepted by the right-hand electrode (cathode) and released by the left-hand electrode (anode). Therefore, Zn reduces copper ions to Cti(s) according to Equation 3.11 for which EceU is positive.
But, for the reverse reaction
\(\mathrm{Cu}(\mathrm{s})+\mathrm{Zn}^{2+}(\mathrm{aq}) \rightarrow \mathrm{Cu}^{2+}(\mathrm{aq})+\mathrm{Zn}(\mathrm{s})\)the emf is negative, i.e., \(E_{\text {cell }}^\theta=-1.1 \mathrm{~V}\) Tire reverse reaction is made possible by applying an external opposite potential to the galvanic cell. As long as the external voltage is less than 1.1V (\(E_{\text {cell }}\), of the Daniell cell) the exception proceeds spontaneously; zinc gets oxidized and Cu2+ ions get reduced deporting copper at the cathode.
Current flows from copper to zinc. When the external potential becomes equal to Ewll, neither do electrons flow through the cell nor does any reaction take place. When EwU is exceeded, the reaction proceeds in the reverse direction# electrons flow from copper to zinc as copper gets oxidized and Zn2+ ions get reduced, deputing zinc at the zinc electrode, which now acts as the cathode. The cell now functions as an electrolytic cell
Half-Cell Potential Of A Daniell Cell
Each compartment consisting of an electrode dipped in the corresponding electrolyte solution is called a half-veil As you already know, the electrode may be positively or negatively charged with respect to the solution, Hence, a potential difference develops between the electrode and the electrolyte, called electrode potential bach half-cell in a cell can therefore be assigned a half-cell potential which is nothing hut the electrode potential The ha I heel I potential could be either oxidation potential when oxidation occurs or reduction potential when reduction occurs, The sum of half-cell potentials gives the cell potential For the Daniel! cell formed of copper and zinc electrodes operating under standard state conditions, the anode or oxidation potential is 0.763 V and the cathode or reduction potential is 0.337 V at 25°C. Therefore, the cell potential \(\left(E_{\mathrm{cell}}^{\Theta}\right) \text { is } 1.1 \mathrm{~V}\)

It is, however, important to note that by convention, the half-reactions are written as reduction actions and the corresponding half-cell potentials, which are both reduction potentials, are considered fir calculating E-cell
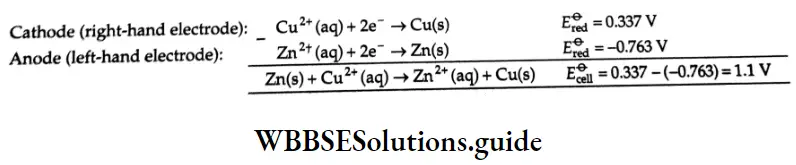
When the species are in their standard states, the half-cell reduction potential is called standard electrode potential. The overall emf of the cell, is then obtained by subtracting the electrode potential of the anode (left-hand electrode) from that of the cathode (right-hand electrode) or simply by the relation
\(E_{\text {cell }}^{\ominus}=E_{\text {right }}^{\ominus}-E_{\text {left }}^{\ominus} \quad \text { (both } E_{\text {right }}^{\ominus} \text { and } E_{\text {left }}^{\ominus} \text { are reduction potentials) }\)For the above Daniell cell,
\(E_{\text {cell }}^{\Theta}=E_{\mathrm{Cu}^{2+} / \mathrm{Cu}}^{\Theta}-E_{\mathrm{Zn}^{2+} / \mathrm{Zn}}^\theta\)\(E_{\mathrm{Zn}^{2+} / \mathrm{Zn}}^{\Theta} \text { (for the reaction } \mathrm{Zn}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Zn} \text { ) }\) is equal to the negative of that of the reverse reation,
i.e., \(\mathrm{Zn} \rightarrow \mathrm{Zn}^{2+}+2 \mathrm{e}^{-}[/lattex] whose potential is represented as [latex]E_{\mathrm{Zn} / \mathrm{Zn}^{2+}}^\theta(0.763 \mathrm{~V})\)
Hence \(E_{\mathrm{Zn}^{2+} / \mathrm{Zn}}^{\Theta}=-0.763 \mathrm{~V}\)
Therefore,
\(E_{\mathrm{cell}}^{\Theta}=0.337-(-0.763) \mathrm{V}\)or, \(E_{\text {cell }}^{\Theta}=1.1 \mathrm{~V}\)
The standard electrode potential of a galvanic cell
Although we can measure the overall cell potential of a galvanic cell, there In no satisfactory method to measure the individual actual potentials of the two electrodes. However, a standard potential can be assigned to one electrode and the other electrode can be assigned a relative value of potentials based on this.
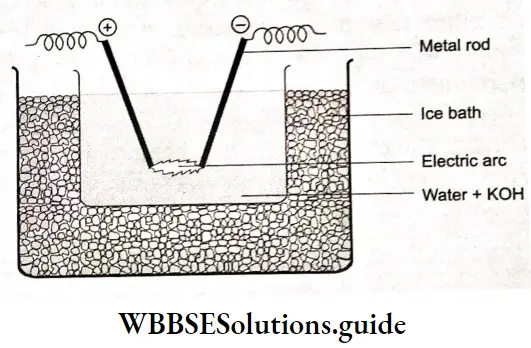
The most commonly used reference electrode is the standard hydrogen electrode (SHE), which is assigned a potential of zero volts at all temperatures. The potentials of all other electrodes are reported relative to that of the SHE.
The standard hydrogen electrode is a gas-ion electrode. In a hydrogen electrode, hydrogen gas is bubbled through a solution of hydrogen ions in which a platinum foil coaled with very finely divided platinum black is dipping partially. Here platinum metal acts as an inert electrode; it does not participate in the reaction—it only provides its surface for the redox reaction. The net ionic equation for reduction in the electrode is
\(2 \mathrm{H}_3 \mathrm{O}^{+}(\mathrm{aq})+2 \mathrm{e}^{-} \rightarrow \mathrm{H}_2(\mathrm{~g}) \div 2 \mathrm{H}_2 \mathrm{O}(\mathrm{I})\)The potential of a gas electrode changes with the pressure of die gas and with the concentration of the other components. If a hydrogen electrode operates at 298 K with hydrogen gas at a pressure of 1 bar bubbling around a platinum foil immersed in a 1M solution of hydronium ions, it is called a standard hydrogen electrode.
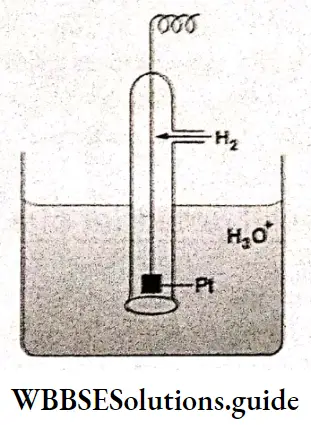
Measurement of the standard electrode potential of a galvanic cell:
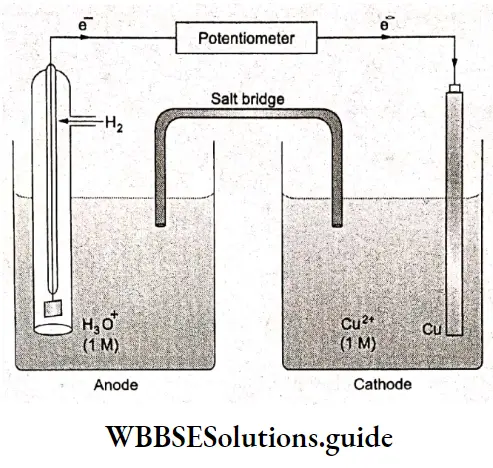
In order to measure the potential of an electrode relative to the SHE, a galvanic cell is constructed with the standard hydrogen electrode and electrode of interest. The cell potential is the potential of the other half-cell, i.e., the electrode whose potential is to be measured.
For example, the potential of a copper electrode can be determined by coupling it with a SHE. The cell diagram is \(\mathrm{Pt}(\mathrm{s})\left|\mathrm{H}_2(1 \mathrm{~atm})\right| \mathrm{H}^{+}(1 \mathrm{M}) \| \mathrm{Cu}^{2+}(1 \mathrm{M}) \mid \mathrm{Cu}(\mathrm{s}) .\). The SHE acts as the anode and the copper electrode as the cathode. The overall redox reaction and the two half-reactions in the cell are
\(\mathrm{Cu}^{2+}(\mathrm{aq})+\mathrm{H}_2(\mathrm{~g})+2 \mathrm{H}_2 \mathrm{O}(\mathrm{l}) \rightarrow \mathrm{Cu}(\mathrm{s})+2 \mathrm{H}_3 \mathrm{O}^{+}(\mathrm{aq})\)Cathode: \(\mathrm{Cu}^{2+}(\mathrm{aq})+2 \mathrm{e}^{-} \rightarrow \mathrm{Cu}(\mathrm{s})\)
Anode: \(\mathrm{H}_2(\mathrm{~g})+2 \mathrm{H}_2 \mathrm{O}(\mathrm{l}) \rightarrow 2 \mathrm{H}_3 \mathrm{O}^{+}(\mathrm{aq})+2 \mathrm{e}^{-}\)
The observed potential for the cell is 0.337 V at 25°C.
\(E_{\text {cell }}^{\Theta}=E_{\text {cathode }}^{\Theta}-E_{\text {anode }}^{\Theta}\) \(0.337=E_{\mathrm{Cu}^{2+} / \mathrm{Cu}}^{\Theta}-E_{\mathrm{H}^{+} / \mathrm{H}_2}^{\Theta}\) \(E_{\mathrm{Cu}^{2+} / \mathrm{Cu}}^{\Theta}-0\)Thus, \(E_{\mathrm{Cu}^{2+} / \mathrm{Cu}}^{\ominus}=0.337 \mathrm{~V}\)
The standard electrode potential of copper, \(E_{\mathrm{Cu}^{2+} / \mathrm{Cu}^{\prime}} \text {, is } 0.337 \mathrm{~V}\) the subscript Cu2+/Cu indicating the reaction \(\mathrm{Cu}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Cu}\) If we wish to determine the standard electrode potential (the reduction potential) of a zinc electrode, the SHE acts as the cathode and the zinc electrode acts as the anode.
The cell diagram is
\(\mathrm{Zn}(\mathrm{s})\left|\mathrm{Zn}^{2+}(1 \mathrm{M}) \| \mathrm{H}^{+}(1 \mathrm{M})\right| \mathrm{H}_2(1 \mathrm{~atm}) \mid \mathrm{Pt}(\mathrm{s})\)The two half-reactions and the overall reaction are as follows.
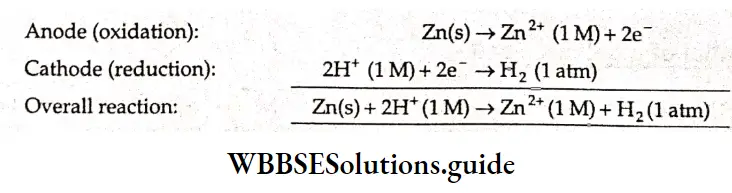
The \(E_{\text {cell }}^{\ominus}\) was found to be 0.76 V.
\(E_{\text {cell }}^{\Theta}=E_{\text {cathode }}^{\Theta}-E_{\text {anode }}^{\Theta}\) \(0.76=E_{\mathrm{H}^{+} / \mathrm{H}_2}^{\Theta}-E_{\mathrm{Zn}^{2+} / \mathrm{Zn}}^{\Theta}\) \(0.76=0-E_{\mathrm{Zn}^{2+} / \mathrm{Zn}}^{\ominus} \Rightarrow E_{\mathrm{Zn}^{2+} / \mathrm{Zn}}^{\Theta}=-0.76 \mathrm{~V}\)Thus tire standard reduction potential of zinc, \(E_{\mathrm{Zn}^{2+} / \mathrm{Zn}^{\prime}} \text { is }-0.76 \mathrm{~V}\)
Lists the values of standard electrode potentials (reduction potentials) for some half-cells.

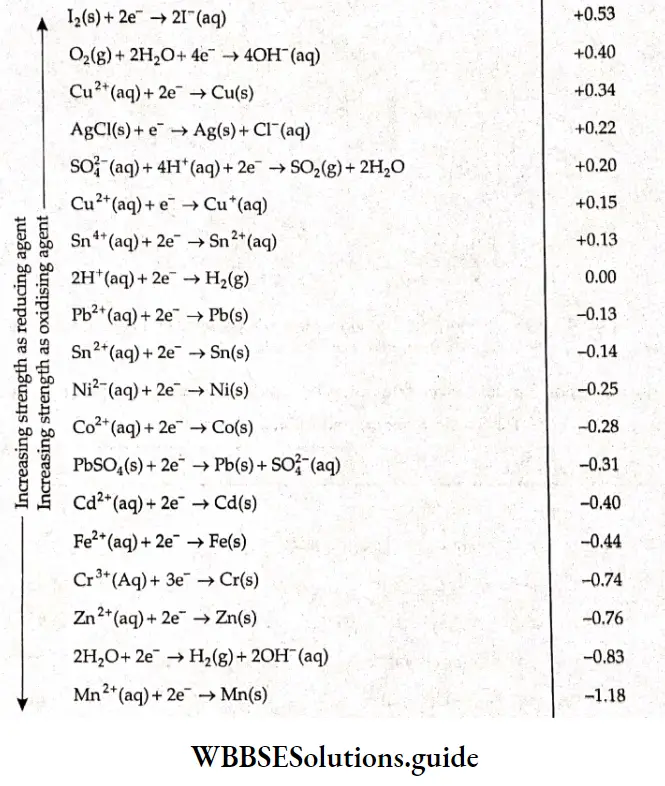
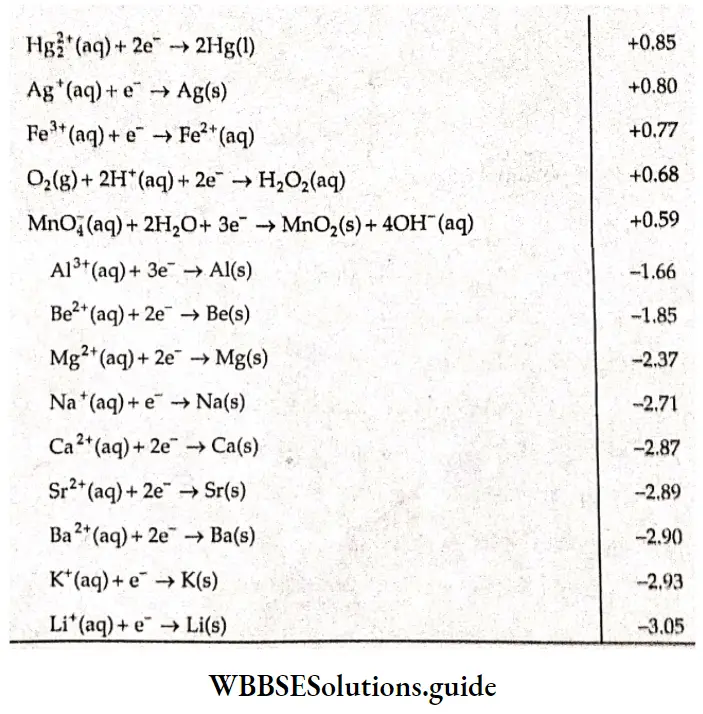
The positive value of standard electrode potential in the example of copper indicates that Cu2+ ions get reduced more easily than the H+ ions. The negative value of standard electrode potential in case of zinc indicates that hydrogen ions can oxidize zinc easily.
The standard electrode potentials are useful in finding out the £Te’ for a combination of different half-cells. It is important to know the following facts about the standard electrode potential values,
1. The \(E^{\Theta}\) values are applicable to the half-cell reactions as read in the forward direction.
2. The half-cell reactions are reversible. A particular electrode can act as anode or cathode, depending on the electrode that is coupled to it,
3. The values of reduction potentials also indicate the tendency of a reaction to occur. The higher the value, the greater is the tendency for reduction, Those electrodes which have a positive electrode potential can be more easily reduced than those having a negative electrode potential. A positive electrode potential indicates a greater tendency to reduce than hydrogen ions.
4. The more positive the value of \(E^{\Theta}\), the greater is the tendency for the substance to be reduced. A look at Table 3.5 shows that P2 has the greatest tendency to get reduced or, in other words, it is the strongest oxidizing agent, Lithium figures at the bottom of the table, indicating that it has the lowest ability to get reduced or the greatest tendency to get oxidized—thus, it is the strongest reducing agent. Thus we see that the relative tendency of metals to get oxidized can be judged from the values of the standard electrode potentials of the corresponding half-cells. The electrochemical series is an arrangement of metal/metal ion electrode potentials in order to decrease the tendency to lose electrons.
5. If any two electrodes are chosen to form a cell, then the electrode that figures first in the table as we move from top to bottom acts ns the cathode and reduction occurs at this electrode. Conversely, the electrode that figures Inter in the table acts as the anode and oxidation occurs at that electrode.
6. Changing the stoichiometric coefficients of a half-cell reaction does not affect the value of \(E^{\Theta}\) as electrode potential is an intensive property.
7. The sign of \(E^{\Theta}\) changes when the reaction is reversed.
Example Calculate the emf of a cell consisting of two half-cells \(\mathrm{Ni}^{2+} / \mathrm{Ni} \text { and } \mathrm{Cd}^{2+} / \mathrm{Cd}\). Given \(E_{\mathrm{Ni}^{2+} / \mathrm{Ni}}^\theta=-0.25 \mathrm{~V}\) concentrations of Nl2+ and Cd2+ are 0.2 M and 0.3 M respectively.
Solution As the reduction potential of the cell Cd2l/Cd is more negative, it has a tendency to undergo oxidation, the two electrode reactions are
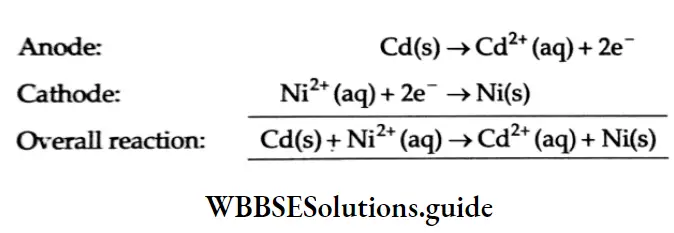
=0.145 V
Types Of Electrodes
The term ‘electrode’ is used in two different ways—one to refer to n wire or some conductor that delivers electrons through the external circuit and the other to refer to a complete half-cell. Let us touch upon the following types of electrodes to illustrate this point.
Gas-ion electrodes A gas-ion electrode consists of an inert collector of electrons such as platinum or graphite in contact with a gas ami a soluble ion, The hydrogen electrode is one example,
\(\mathrm{Pt}^{\mathrm{t}}\left|\mathrm{H}_2(\mathrm{~g})\right| \mathrm{H}^{+}\)We can also have a chlorine electrode, represented by
\(\mathrm{Cl}_2\left|\mathrm{Cl}^{-}\right| \text {graphite }\)Metal-metal ion electrodes A metal strip or rod dipped in a solution containing the metal ions constitutes a metal-metal ion electrode. The Zn2+ /Zn and Cu2+ /Cu electrodes of the Daniell cell are examples. Metal-insoluble salt-anion electrode Such an electrode consists of a bar of metal immersed in a solution containing a solid insoluble salt of the metal and anions of the salt.
The silver-silver chloride electrode, \(\mathrm{Cl}^{-}|\mathrm{AgCl}(\mathrm{s})| \mathrm{Ag}(\mathrm{s})\), and the calomel electrode, \(\mathrm{Cl}^{-}\left|\mathrm{Hg}_2 \mathrm{Cl}_2\right| \mathrm{Hg}\) are two examples.
Oxidation-reduction electrodes Although electrodes generally involve either oxidation or reduction in their operation, there are certain electrodes called oxidation-reduction electrodes. Such electrodes have an inert metal collector, usually platinum dipping in a solution that contains two soluble species in different states of oxidation. An example is the ferric-ferrous ion electrode.
Electrical Work And Free Energy
The chemical reaction in a galvanic cell proceeds spontaneously in the forward direction obeying laws of chemical equilibrium. Electrical work can be obtained using the galvanic cell and the total amount of energy available from a particular cell depends on the cell’s potential and the number of electrons involved. Let us now see how E^u and AGe are related. The unit of potential difference is the volt.
The work done to move a charge across a potential difference is given by the product of the charge and the potential difference. The unit of electrical work is the joule. Thus, one volt (V) is the potential difference required to impart one joule 0) of energy to a charge of one coulomb (C). In other words, one joule of energy is available when a charge of one coulomb passes between electrodes having a unit potential difference.
1J = 1V x 1C
In this context, it is important to recall that in a galvanic cell, the electrons flow in the direction determined by the potential difference between the two electrodes. When the two electrodes acquire the same potential, there is no passage of current. More work is available when more charge is moved between the electrodes.
The quantity of charge flowing through an electrical circuit connected to a galvanic cell equals the product of the number of moles of electrons (n) flowing through the circuit and the Faraday constant. The Faraday constant is the electrical charge contained in 1 mol of electrons. Experiments have shown that 1 faraday is equivalent to 96,487 coulombs.
Thus,
\(1 F=96,487 \frac{\mathrm{C}}{\mathrm{mol} \mathrm{e}^{-}} \text {or } 96,487 \frac{\mathrm{J}}{\mathrm{V} \mathrm{mol} \mathrm{e}^{-}} \text {and } C=n F\)The electrical work (Wcle) done on the circuit is equal to the product of this charge and the electromotive force pushing it. When the work is expressed as the work done by the cell, a negative sign is introduced.
\(W_{\text {ele }}=-C E_{\text {cell }}=-n F E_{\text {cell }}\)where n is the number of electrons transferred, F is the Faraday constant and Ecell is the cell potential. A negative cell potential means that energy is consumed while a positive potential means that work is done by the cell. The maximum amount of electrical work that can be done by the system is equal to the maximum amount of useful work. Hence,
\(W_{\max }=W_{\text {ele }}=-n F E_{\text {cell }}\)A cell can do no work when the potential is zero (or the reaction is at equilibrium).
When the current produced by the cell is used to do work, the energy of the system (the cell) is reduced and the sign of work done is negative but the cell potential is positive. Thus the magnitude of the cell potential and the maximum amount of work Wmax available from the cell have opposite signs.
As you already know, the Gibbs free energy for a system (AG) is equal to the maximum possible useful work done by the system, Equation 3.12 can be further modified as
\(\Delta G=W_{\max }=-n F E_{\text {cell }}\)where ΔG is the Gibbs energy change for the reaction,
The free energy of the system (here cell), therefore, decreases (i.e., has a net negative value) when the system does some useful work. The actual amount of work that a cell can do is, in fact, less than the calculated maximum amount of work because some energy is converted into heat owing to the resistance in the circuit.
When the concentrations of the reacting species are unity, the corresponding free energy change, and cell potential values are standard state values.
\(\Delta G^{\ominus}=-n F E_{\text {cell }}^{\ominus}\)Thus, cell potential can be used to calculate \(\Delta G \text { or } \Delta G^{\ominus}\) for a process.
Example 1. Calculate the standard free energy change for the reaction occurring in the following cell.
\(\mathrm{Zn}(\mathrm{s})\left|\mathrm{Zn}^{2+}(\mathbf{1} \mathrm{M}) \| \mathrm{Cu}^{2+}(\mathbf{1} \mathrm{M})\right| \mathrm{Cu}(\mathrm{s})\)
\(\text { Given } E_{\mathrm{Zn}^{2+} / \mathrm{Zn}}^\theta=-0.76 \mathrm{~V} ; E_{\mathrm{Cu}^{2+} / \mathrm{Cu}}^\theta=0.34 \mathrm{~V} ; F=96500 \mathrm{C} \mathrm{mol}^{-1}\)
Solution:
Since \(\Delta G^{\ominus}=n F E_{\text {cell }}^{\Theta}\)
the number of electrons involved in the reaction (n) = \(2 \text { and } E_{\text {cell }}^{\Theta}=\underset{\substack{\text { cathode } \\(\mathrm{RHE})}}{E_{\text {and }}^{\Theta}}-\underset{\text { (LHE) }}{E_{\text {anode }}}=0.34-(-0.76)\)
\(E_{\text {cell }}^{\ominus}=0.34+0.76=1.10 \mathrm{~V}\)∴\(\Delta G^{\ominus}=-2 \times 96500 \times 1.1=-212300 \mathrm{~J}=-212.3 \mathrm{~kJ} \quad(1000 \mathrm{~J}=1 \mathrm{~kJ})\)
Example 2. Calculate the maximum possible electrical work that can be obtained from the following cell under standard conditions of temperature and pressure.
\(\mathrm{Zn} / \mathrm{Zn}^{2+}(\mathrm{aq}) \| \mathrm{Sn}^{2+}(\mathrm{aq}) \mid \mathrm{Sn}\)
Given \(E_{\mathrm{Zn}^{2+} / \mathrm{Zn}}^{\Theta}=-0.76 \mathrm{~V} \text { and } E_{\mathrm{Sn}^{2+} / \mathrm{Sn}}^{\Theta}=-0.14 \mathrm{~V}\)
Solution:
Electrical work done, \(W_{\text {ele }}=\Delta G^\theta\) under standard conditions and \(\Delta G^{\Theta}=-n F E_{\text {cell }}^{\Theta}\)
Also \(E_{\mathrm{cell}}^{\Theta}=E_{\mathrm{Sn}^{2+} / \mathrm{Sn}}^{\Theta}-E_{\mathrm{Zn}^{2+} / \mathrm{Zn}}^{\Theta}=-0.14-(-0.76)\)
\(E_{\text {cell }}^{\ominus}=0.62 \mathrm{~V} ; n=2\) (two electrons are involved in the cell reaction).
∴ \(\Delta G^{\ominus}=W_{\text {ele }}=-2 \times 96500 \times 0.62=-1,19,660 \mathrm{~J}\)
Thus, 119.7 kJ of work can be obtained from this cell.
It is important to note that since ΔG is an extensive thermodynamic property and the value depends on n, it changes with the change of stoichiometric coefficients. For example, the values of ΔG for the reactions
\(\mathrm{Zn}(\mathrm{s})+\mathrm{Cu}^{2+}(\mathrm{aq}) \rightarrow \mathrm{Zn}^{2+}(\mathrm{aq})+\mathrm{Cu}(\mathrm{s})\)and \(2 \mathrm{Zn}(\mathrm{s})+2 \mathrm{Cu}^{2+}(\mathrm{aq}) \rightarrow 2 \mathrm{Zn}^{2+}(\mathrm{aq})+2 \mathrm{Cu}(\mathrm{s})\)
\(\text { are }-2 F E_{\text {cell }} \text { and }-4 F E_{\text {cell }} \text { respectively. }\)Effect Of Concentration On Cell Potential (Nernst Equation)
We may not always measure the potential of a cell under standard conditions. What happens when the concentrations of the species involved are not unity? The cell potential of the cell changes with the change in concentrations of the species
Let us consider a galvanic cell reaction of the general type
\(a \mathrm{~A}+b \mathrm{~B} \rightleftharpoons c \mathrm{C}+d \mathrm{D}\)The reaction quotient Q is given by
\(Q=\frac{[\mathrm{C}]^c[\mathrm{D}]^d}{[\mathrm{~A}]^a[\mathrm{~B}]^b}\)where the square brackets indicate the concentrations of the corresponding species and all quantities are raised to the power of the respective stoichiometric coefficients. Note that products appear in the numerator and reactants in the denominator.
The free energy change AG for the reaction is given by
\(\Delta G=\Delta G^{\Theta}+R T \ln \left[\frac{[\mathrm{C}]^c[\mathrm{D}]^d}{[\mathrm{~A}]^a[\mathrm{~B}]^b}\right]\)Substituting \(\Delta G \text { and } \Delta G^{\ominus} \text { in terms of } E_{\text {cell }} \text { and } E_{\text {cell, }}^{\ominus} \text { we get }\)
\(-n F E_{\text {cell }}=-n F E_{\text {cell }}^\theta+R T \ln Q\).
\(E_{\text {cell }}=E_{\text {cell }}^{\Theta}-\frac{R T}{n F} \ln Q\).
Here n is the number of moles of electrons exchanged in the cell reaction. Equation (3-13) is known as the Nemst equation after the German chemist and physicist W H Nemst, who was awarded the Nobel Prize in Chemistry in 1920.
By converting the natural logarithm to the base 10 and at a temperature of 298 K, and substituting for the values of R and F, the above equation can be written as
\(E_{\text {cell }}=E_{\text {cell }}^{\ominus}-\frac{0.05916}{n} \log Q\)For a half-cell reaction
\(\mathrm{M}^{n+}(\mathrm{aq})+n \mathrm{e}^{-} \rightarrow \mathrm{M}(\mathrm{s})\)the electrode potential at any concentration measured by connecting it to an SHE can be represented by
\(E_{\mathrm{M}^{n+} / \mathrm{M}}=E_{\mathrm{M}^{n+} / \mathrm{M}}^{\Theta}-\frac{R T}{n F} \ln \frac{[\mathrm{M}]}{\left[\mathrm{M}^{n+}\right]}\)Since M is a solid, its concentration is taken as unity and the above equation then becomes
\(E_{\mathrm{M}^{n+} / \mathrm{M}}=E_{\mathrm{M}^{n+} / \mathrm{M}}^{\Theta}-\frac{R T}{n F} \ln \frac{1}{\left[\mathrm{M}^{n+}\right]}\)Consider the example of the Daniell cell; the cell potential is given by
\(E_{\text {cell }}=E_{\text {cell }}^{\Theta}-\frac{0.05916}{2} \log \frac{\left[\mathrm{Zn}^{2+}\right][\mathrm{Cu}]}{[\mathrm{Zn}]\left[\mathrm{Cu}^{2+}\right]}\)Since the activities of solid copper and zinc are unity,
\(E_{\text {cell }}=E_{\text {cell }}^\theta-\frac{0.05916}{2} \log \frac{\left[\mathrm{Zn}^{2+}\right]}{\left[\mathrm{Cu}^{2+}\right]}\)or \(E_{\text {cell }}=E_{\text {cell }}^{\Theta}+\frac{0.05916}{2} \log \frac{\left[\mathrm{Cu}^{2+}\right]}{\left[\mathrm{Zn}^{2+}\right]}\)
As you can see, the value of the cell potential depends on the concentrations of both Cu2+ and Zn2+. It increases with an increase in the concentration of Cu2+ and decreases with an increase in the concentration of Zn2+.
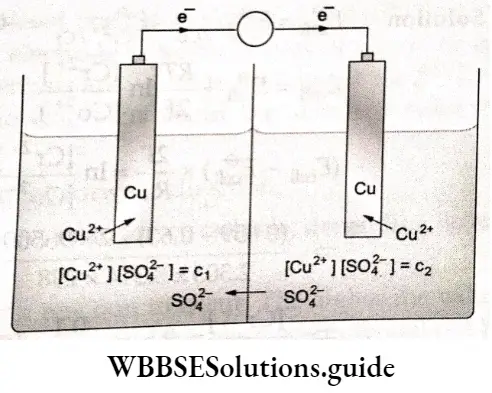
In case of cells with electrodes of some type dipping in same type of elect roly tie solutions hut wllli different concentrations (known as consent ml ion cells), (lie standard potentials of the electrodes cancel each other and the cell potential is given in terms of the concentration of electrolytic solutions, for example, In the concentration cell,
\(\mathrm{Cu}\left|\mathrm{CuSO}_4\left(c_1\right) \| \mathrm{CuSO}_4\left(c_2\right)\right| \mathrm{Cu}\) the cell potential is given by where q and c2 are the concentrations of the two solutions.
where c1 and c2 are the concentrations of the two solutions.
Cell Potential And Equilibrium Constant
The \(\Delta G^\theta\) of a reaction is related to the equilibrium constant K as
\(\Delta G^\theta=-R T \ln K\)but \(\Delta G^\theta=-n F E_{\text {cell }}^\theta\)
Hence,
\(-n F E_{\mathrm{cell}}^{\Theta} \equiv-R T \ln K\) \(E_{\text {cell }}^\theta=\frac{R T}{n F} \ln K\)If the standard potential of a cell is known then the equilibrium constant of a reaction taking place in that ceil can be obtained from Equation (3.15). On substituting the values of R and P and conversion to common logarithm at 298 K, we get
\(E_{\text {oell }}^{\Theta}=\frac{0.05916}{n} \log K\)In an electrochemical cell, the voltage as read on a voltmeter drops gradually and becomes zero at some point of time. This is because as the reaction in the cell proceeds, the concentrations of reactants keep decreasing and those of products increase till equilibrium is attained, when there is no change in the concentrations of either. At that time, cell potential is zero.
Example 1. Calculate the end of a cell consisting of two half-cells \(\mathrm{Al}^{3+} / \mathrm{Al} \text { and } \mathrm{Ag}^{+} / \mathrm{Ag}\). Given that \(E_{\mathrm{Al}^{3 *} / \mathrm{Al}}^6=-1.66 \mathrm{~V}\) and \(E_{\mathrm{Ag}^{+} / \mathrm{Ag}^{\Theta}}^{\Theta}=0.8 \mathrm{~V} \text { at } 298 \mathrm{~K}\)
Solution:
The standard reduction potential of the half-cell reactions are
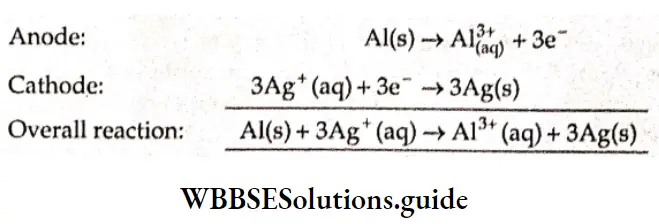
= 2.46 + 0.104
= 2.564 V.
Example 2. A cell is made up of two electrodes \(\mathrm{Cr}^{2+} / \mathrm{Cr} \text { and } \mathrm{Co}^{2+} / \mathrm{Co}\). The standard reduction potentials are \(E_{\mathrm{Cr}^{2+} / \mathrm{Cr}}^{\Theta}=-0.91 \mathrm{~V} ; E_{\mathrm{Cu}^{2+} / \mathrm{Co}}^{\Theta}=-0.28\) at 298 K. If the cell is 0.659 V and the concentration of Cr2+ 0.1 M, find the concentration of CO2+.
Solution:
= 0.01 M.
Example 3. For a reaction, K = 1.8x 10 7 at 300 K. What is the value of \(\Delta G^{\ominus}\) at this temperature?
Solution:
Example 4. Calculate the equilibrium constant of the reaction
\(\mathrm{Ni}(\mathrm{s})+\mathrm{Cu}^{2+}(\mathrm{aq}) \rightarrow \mathrm{Cu}(\mathrm{s})+\mathrm{Ni}^{2+}(\mathrm{aq})\)
\(E_{\mathrm{Ni}^{2+} / \mathrm{Ni}}^{\Theta}=-0.25 \mathrm{~V}, E_{\mathrm{Cu}^{2+} / \mathrm{Cu}}^{\Theta}=0.34 \mathrm{~V}\);
\(R=8.314 \mathrm{~J} \mathrm{~K}^{-1} \mathrm{~mol}^{-1}, F=96,500 \mathrm{C} \mathrm{mol}^{-1}\)
Solution:
and \(E_{\mathrm{cell}}^{\ominus}=E_{\mathrm{Cu}^{2+} / \mathrm{Cu}}^{\Theta}-E_{\mathrm{Ni}^{2+} / \mathrm{Ni}}^{\ominus}=0.34-(-0.25)=0.59 \mathrm{~V} \text { and } n=2\)
∴ \(\Delta G^{\ominus}=-2 \times 96500 \times 0.59=-113,870 \mathrm{~J} \mathrm{~mol}^{-1}\)
\(\ln K=-\frac{\Delta G^\theta}{R T}=\frac{113,870}{8.314 \times 298}=45.96\)2.303 log K = 45.96
log K = 19.96
K = antilog (19.96)
= 9.12 x 10 19.
Electrolysis and electrolytic cells
When electrodes (metallic conductors) are dipped in a solution of an electrolyte and a sufficient potential difference, of the order of several volts, is applied across the electrodes, chemical reactions are observed at the electrodes. This process is known as electrolysis.
Electrolysis is observed in an electrolytic cell (or an electrochemical cell) in which a nonspontaneous reaction is driven by an external source of current. When electrolysis occurs in an electrolytic cell the electrode that is charged positively by the applied potential resulting in a deficit of electrons is called the anode and that chareed negatively with an excess of electrons is called the cathode.
In this context, it is also important to distinguish between inert electrodes and reacting electrodes. Inert electrodes, usually platinum wires, serve only to transfer electrons to and from the solution. Reacting electrodes chemically enter into an electrode reaction. Metals except platinum are examples of reacting electrodes and contribute metal ions to the solution.
The two major electrode reactions that occur in electrolysis are oxidation and reduction. At the anode, loss of electrons (oxidation) occurs whereas at the cathode electrons are introduced (by the external circuit) and reduction occurs.
Electrolysis was studied extensively by Michael Faraday in 1820. He observed that the amount of charge passed through an electrolyte was quantitatively related to the amount of products formed at the electrodes.
These quantities axe conveniently related by introducing the faradav unit of charge with the symbol Fr defined as the charge of 3 mol, or an Avogadro number, of electrons. We have
1F = NA x (electronic charge)
= \(6.023 \times 10^{23} \times 1.602 \times 10^{-19}=96,485 \mathrm{C} \mathrm{mol}^{-1}\)
Based on his experimental findings, Faraday gave two laws of electrolysis, which are as follows.
First law:
The amount of a chemical substance liberated at an electrode during electrolysis is directly proportional to the quantity of electricity passed through the cell.
Second law:
The amounts of different substances produced by a given quantity of electricity (passing through the cell) are proportional to the molar masses of the substances.
The laws are applicable to molten electrolytes as well as to solutions of electrolytes and are independent of temperature, pressure or nature of the solvent. One important application of electrolysis is in the extraction of metals such as lithium, sodium, magnesium, aluminum and calcium.
Electrolysis of molten sodium chloride:
The commercial electrolysis of molten sodium chloride is carried out in a Down’s cell. This cell consists of an airtight vessel containing molten sodium chloride, two inert electrodes and a porous separator screen that permits diffusion of ions through the cell but prevents intermixing of the reactants and products. At the cathode, sodium ions get reduced to sodium metal At the anode the chloride ions get oxidized to chlorine gas. The two half-reactions are as follows.
Cathode: \(\mathrm{Na}^{+}(\mathrm{l})+\mathrm{e}^{-} \rightarrow \mathrm{Na}(\mathrm{s})\)
Anode: \(2 \mathrm{Cl}^{-}(\mathrm{l}) \rightarrow \mathrm{Cl}_2(\mathrm{~g})+2 \mathrm{e}^{-}\)
To balance the electrons, by multiplying Equation 3.17 by 2, we get
\(2 \mathrm{Na}^{+}(\mathrm{l})+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{Na}(\mathrm{s})\)The net reaction is obtained by adding Equations 3.18 and 3.19.
\(2 \mathrm{Na}^{+}(\mathrm{l})+2 \mathrm{Cl}^{-}(\mathrm{l}) \rightarrow 2 \mathrm{Na}(\mathrm{s})+\mathrm{Cl}_2(\mathrm{~g})\)For this process, a voltage of about 5 V is applied with a current of thousands of amperes. Li, Mg, and Ca are obtained by the electrolysis of their respective chlorides—they are produced at the cathode.
Quantitative aspects To reduce one sodium ion, one electron is required. Therefore, to reduce one mole of sodium ions (Na~), one mole of electrons is required, and one mole of sodium ions gives rise to one gram atom, i.e., 23 of sodium metal. Thus, on passing one mole of electrons (one faraday of electricity), 23 g of sodium metal is produced. At the same time, if one faraday of electricity is removed from the anode, 35.4 g of chloride ions (CT) is discharged, Le., 0.5 mol of Cl2 gas is produced according to the reaction
\(\mathrm{Cl}^{-}(\mathrm{l}) \rightarrow \frac{1}{2} \mathrm{Cl}_2(\mathrm{~g})+\mathrm{e}^{-}\)The charge carried by n moles of electrons is
Q = nF.
If the oxidation reaction is
\(2 \mathrm{Cl}^{-}(\mathrm{l}) \rightarrow \mathrm{Cl}_2(\mathrm{~g})+2 \mathrm{e}^{-}\)then the total charge carried by the electrons is Q = 2F. Therefore, on passing two faradays of electricity, one mole of Cl2(g) is produced. The charge passed through the cell during electrolysis is equal to the product of current (1) in amperes and time (t) in seconds.
Q = It.
Example 1. Calculate the amount of product formed at the two electrodes when a current of 0334 A is passed through a cell constructed for the electrolysis of calcium chloride for 2 h.
Given: Atomic mass of Ca = 40.08 and that of Cl = 35.4.
Solution:
The only species that we get from CaCl2 are Ca2+ and Cl ions. Hence it is easy to guess that Cl ions will get oxidised while Ca2+ ions will get reduced.
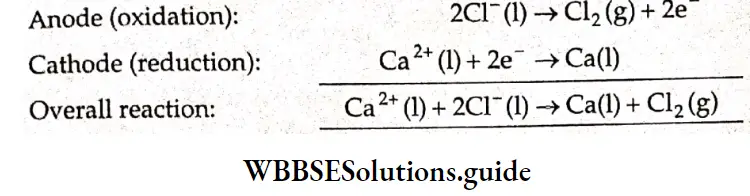
Amount of charge passed through the cell,
\(Q=I t=0.334 \mathrm{~A} \times 2 \mathrm{~h} \times \frac{3600 \mathrm{~s}}{1 \mathrm{~h}} \times \frac{1 \mathrm{C}}{1 \mathrm{~A} \cdot \mathrm{s}}=2404.8 \mathrm{C}\)Since 1 mol of e- = 96,487 C and 2 mol of electrons is required to reduce 1 mol of Ca2+ ions, the mass of Ca formed at the cathode is calculated as follows.
Amount of Ca = \(2404.8 \mathrm{C} \times \frac{1 \mathrm{~mol} \mathrm{e}^{-}}{96,487 \mathrm{C}} \times \frac{1 \mathrm{~mol} \mathrm{Ca}}{2 \mathrm{~mol} \mathrm{e}^{-}} \times \frac{40.08 \mathrm{~g} \mathrm{Ca}}{1 \mathrm{~mol} \mathrm{Ca}}=0.5 \mathrm{~g}\)
For the anodic reaction, 2 mol of electrons is required to oxidize Cl ions or to produce 1 mol of Cl2 gas.
Amount of Cl2 (in grams) = \(2404.8 \mathrm{C} \times \frac{1 \mathrm{~mol} \mathrm{e}^{-}}{96,487 \mathrm{C}} \times \frac{1 \mathrm{~mol} \mathrm{Cl}_2}{2 \mathrm{~mol} \mathrm{e}^{-}} \times \frac{2 \times 35.4}{1 \mathrm{~mol} \mathrm{Cl}_2}=\mathbf{0 . 8 8 2}\)
Example 2. How much charge is required to be passed through a cell containing a Cd2+/ Cd electrode so that 0.5 mol of Cd is deposited at the electrode?
Solution:
The reaction occurring in the cell is
\(\mathrm{Cd}_{(\mathrm{aq})}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Cd}(\mathrm{s})\)1 mol of Cd (s) requires 2 mol of electrons.
∴ 0.5 mol Cd (s) requires 1 mol of electrons.
Charge on 1 mol of electrons = \(1.6023 \times 10^{-19} \mathrm{C} \times 6.023 \times 10^{23} \text { electron } \mathrm{mol}^{-1}=96,485 \mathrm{C}\)
Hence 96,485 C of charge should be passed for the deposition of 0.5 mol of Cd.
Example 3. How much charge is required to be passed through a cell containing a Cu2+/Cu electrode so that 25 g of Cu (atomic weight = 63.5) gets deposited at the electrode?
Solution:
1 mol of Cu2+ requires 2 mol of electrons (v charge of 1 mol of electrons = 96,485 C)
= 2x 96485 C ≅ 2 x 96500 C
= 193, 000 C.
For 63.5 g (1 mol) 193,000 C of charge is required.
∴ For 25 g of Cu \(\) of charge is required.
= 75,984 C of charge is required.
Example 4. Find the mass of Al deposited according to the reaction \(\mathrm{Al}^{3+}+3 \mathrm{e}^{-} \rightarrow \mathrm{Al}\), given that a current of 4 A is passed for 50 min. Atomic mass of AI = 27.
Solution:
Since \(\frac{193,000}{63.5} \times 25 \mathrm{C}\) = y, total charge passed = 4x50x60As = 12,000 C.
3 mol of electrons or 3 x 96,500 C of charge deposits 1 mol of Al.
∴12,000 C of charge will deposit \(\frac{12,000}{3 \times 96,500}\) = 0.0414 mol of AI = 0.0414 x 27 g of AI = 1.117 g of AI.
Example 5. Find the amount of Na deposited at an electrode due to the reaction \(\mathrm{Na}^{+}+\mathrm{e}^{-} \rightarrow \mathrm{Na}\) when passed through molten NaCl for 80 min (atomic mass of Na = 23).
Solution:
According to the reaction, 1 mol of electrons is required for depositing 1 mol of Na.
Charge on 1 mol of electrons = 96,500 C.
Charge passed = 2 x 80 x 60 = 9600 C.
∴ amount of Na deposited = \(\frac{9600}{96,500} \times 1 \approx 0.1 \mathrm{~mol}\) = 0.1 x 23 = 2.3 g.
Example 6. How much time is required for the deposition of 15.875 g of Cu according to the electrode reaction \(\mathrm{Cu}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Cu}\) when 4 A of current is passed through the cell? The atomic mass of Cu = 63.5.
Solution:
According to the electrode reaction, for 1 mol of Cu, 2 mol of electrons is required, i.e., for 63.5 g of Cu, 2 x 96,500 C of charge is required.
For 15.875 g of Cu, \(\frac{2 \times 96,500}{63.5}\) x 15.875 = 48,250 C of charge is required.
Charge = current x time.
∴ time required for the deposition of 15.875 g of Cu
\(=\frac{48,250}{4}=12,062.5 \mathrm{~s}=\frac{12,062.5}{3600} \text { hours }\)= 3.35 hours.
Products Of Electrolysis
As the amount of the substance or products liberated at the electrode depends on the amount of electricity passed [ through the cell, the products of electrolysis depend on the state of the substance (i.e., molten or aqueous) and the nature of the electrodes being used in the electrolysis. For instance, the electrolysis of molten sodium chloride yields sodium metal and chlorine gas. But what will happen if an aqueous solution of sodium chloride is used? f Let us discuss this case along with some more examples.
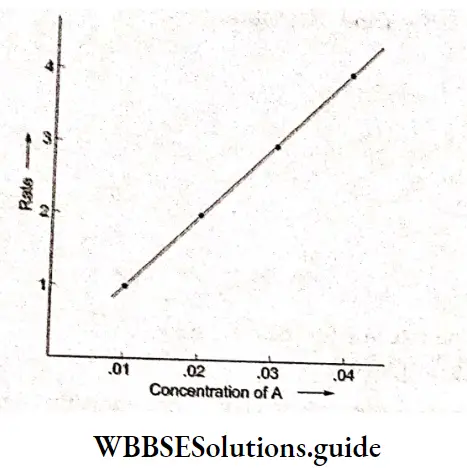
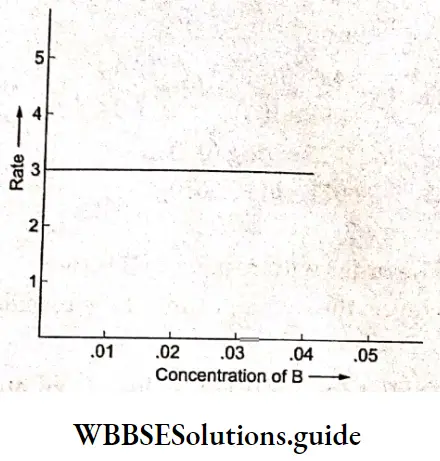
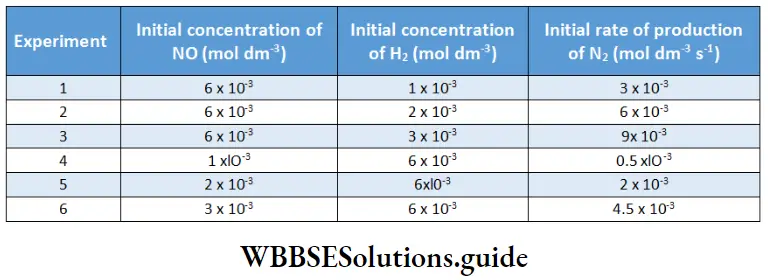
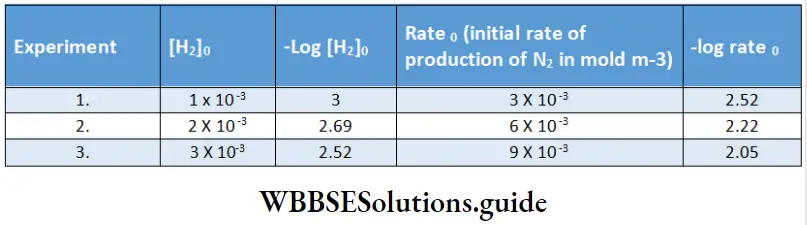
Electrolysis of aqueous sodium chloride:
In tire electrolysis of an aqueous solution of sodium chloride, more than one reaction is possible at each electrode. At the cathode, the sodium ion as well as water can be reduced.
\(\mathrm{Na}^{+}+\mathrm{e}^{-} \rightarrow \mathrm{Na} \quad E_{\text {red }}^{\Theta}=-2.714 \mathrm{~V}\) \(2 \mathrm{H}_2 \mathrm{O}+2 \mathrm{e}^{-} \rightarrow \mathrm{H}_2+2 \mathrm{OH}^{-} \quad E_{\mathrm{red}}^{\ominus}=-0.828 \mathrm{~V}\)Tire species with a higher reduction potential can get more easily reduced. Between sodium ions and water, water has a larger reduction potential and hence the reaction involving water is the one favored at the cathode.
At the anode, the chloride ion as well as water can be oxidised. In order to optimise the production of chlorine, n concentrated solution of sodium chloride is used.
The reactions occurring at the two electrodes and the overall reaction are as follows.
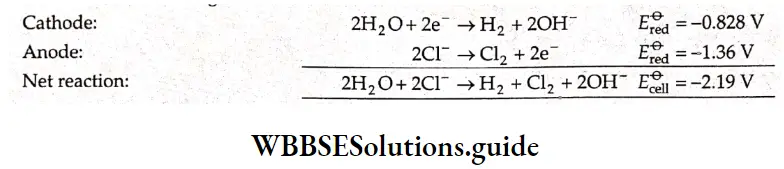
Because the sodium ions remain unchanged, more and more sodium hydroxide is formed in the solution as electrolysis proceeds. Hence the electrolysis of an aqueous solution of sodium chloride can be used for the commercial production of H2 gas, Cl2 gas and NaOH. This is possible by using currents of 10,000 – 60,000 A at about 3.8 V.
Electrolysis of aqueous HCI:
When an aqueous solution of hydrochloric acid is electrolyzed, hydronium ions are reduced to hydrogen gas at the cathode.
\(2 \mathrm{H}_3 \mathrm{O}^{+}+2 \mathrm{e}^{-} \rightarrow \mathrm{H}_2+2 \mathrm{H}_2 \mathrm{O}\)At the anode, two oxidation reactions are possible.
\(2 \mathrm{Cl}^{-} \rightarrow \mathrm{Cl}_2+2 \mathrm{e}^{-} \quad E_{\mathrm{ov}}^{\Theta}=-1.36 \mathrm{~V}\) \(6 \mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{O}_2+4 \mathrm{H}_3 \mathrm{O}^{+}+4 \mathrm{e}^{-} \quad E_{\mathrm{ox}}^{\Theta}=-1.23 \mathrm{~V}\)The oxidation potentials of these two reactions are comparable and both can occur with equal ease. However, which one of them occurs or whether both of them occur depends upon the concentration of chloride ions in the solution.
In a very dilute solution of HCl, H2O is electrolysed and oxygen is released at the anode.
In concentrated solutions of HCl, chloride ions are reduced to form chlorine gas at the anode (Equation 3.20). The reactions are as follows.
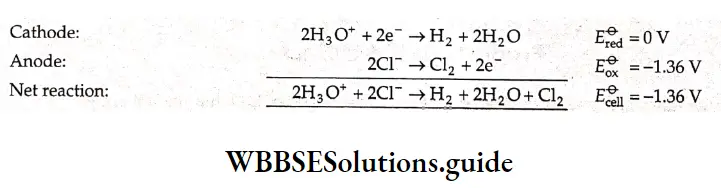
A voltage of 1.36 V (or more) has to be applied for the above reaction to occur at standard state conditions. At moderate concentrations of HCl, both the reactions—the electrolysis of water and the oxidation of chloride ions—occur.
Electrolytic deposition of metals:
Chrome plating, bronzing, and refining of copper, all employ electrolysis.
In bronzing, a metallic strip of copper is used as the anode. Copper is oxidized to form copper ions.
\(\mathrm{Cu} \rightarrow \mathrm{Cu}^{2+}+2 \mathrm{e}^{-}\)The cathode may be any conducting material or a nonconducting material dusted with graphite powder to make it conduct. The copper ions formed are reduced at the cathode and copper is deposited there.
\(\mathrm{Cu}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Cu}\)Thus, the material used as the cathode is bronzed.
If copper is used as the cathode, refined copper is obtained. Copper is purified electrochemically to improve its conductivity. The process is called electrorefining. Impure copper is made of the anode whereas the cathode is pure copper. During electrolysis, the less active metals such as gold and silver, which do not get as easily oxidized as copper settle down as ‘anode mud’, at the bottom of the cell. Impurities
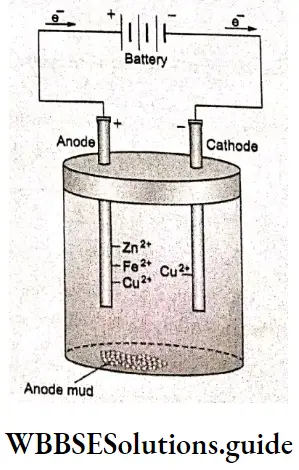
such as iron and zinc, which are more easily oxidized than copper, get oxidized at the anode and go into the solution as ions. When the potential difference between the electrodes is carefully controlled, copper is oxidized to copper ions at the anode and Fe2+ and Zn2+ are not reduced at the cathode. Only Cu2+ ions are reduced to form metallic copper, and deposit at the cathode (which can also be a metal other than copper). Thus pure copper is obtained at the cathode.
Corrosion
On exposure to air and water, the surfaces of many metallic objects, in particular those made of iron, rust. Rusting is nothing but corrosion. The presence of an electrolyte accelerates corrosion, which is an electrochemical process. When iron is in contact with even a droplet of water, it is oxidized and releases electrons. These electrons, on coming into contact with air, reduce oxygen forming the hydroxide ion. Thus two half-cells, whose reactions are given below, are created.
Anode: \(\mathrm{Fe} \rightarrow \mathrm{Fe}^{2+}+2 \mathrm{e}^{-}\)
Cathode: \(\mathrm{O}_2+2 \mathrm{H}_2 \mathrm{U}+4 \mathrm{e}^{-} \rightarrow 4 \mathrm{OH}^{-}\)
The Fe2+ and OH- ions thus formed diffuse together to form insoluble iron (2) hydroxide.
\(\mathrm{Fe}^{2+}(\mathrm{aq})+2\left(\mathrm{OH}^{-}\right)(\mathrm{aq}) \rightarrow \mathrm{Fe}(\mathrm{OH})_2(\mathrm{~s})\)Ferrous hydroxide is rapidly oxidized by oxygen to rust with an approximate composition of Fe2O3. H2O.
Corrosion may cause severe damage to buildings, ships, bridges and all objects made of metals, especially iron. Therefore prevention of corrosion is very important to avoid accidents such as the collapse of a bridge or the nonfunctioning of a part in an engine. The simplest of the methods is to prevent the metallic surface from coming in contact with air by coating the surface with paint or other chemicals.
Corrosion can also be prevented by coating iron with grease or asphalt or with ceramic enamel as used in sinks, refrigerators, etc. A tough coating of Fe3O4 is obtained by exposing iron to superheated steam. We can use alloys of iron such as stainless steel, which is corrosion-resistant.
Metals such as chromium, tin or zinc afford a more durable surface coating for iron than paint. The steel used in automobiles, for example, is coated with zinc by dipping it into a bath of molten zinc. This process is known as galvanization. Zinc is more easily oxidized than iron and whenever oxidation occurs, zinc is oxidized rather than iron.
\(\mathrm{Fe}^{2+}(\mathrm{aq})+2 \mathrm{e}^{-} \rightarrow \mathrm{Fe}(\mathrm{s}) \quad E^{\Theta}=0.45 \mathrm{~V}\) \(\mathrm{Zn}^{2+}(\mathrm{aq})+2 \mathrm{e}^{-} \rightarrow \mathrm{Zn}(\mathrm{s}) \quad E^{\ominus}=-0.76 \mathrm{~V}\)Any oxidation, if at all, of iron is reversed immediately as Zn can reduce Fe2 to Fe2-.
Instead of covering the entire surface of a metal with another metal, a simple electrical contact with a second metal can save the first one from corroding. This is called cathodic protection.
Cathodic protection is an important application of electrochemistry used for protecting iron or steel. It is used in underground pipelines that are in contact with soil. The iron present as a component is connected by a wire to a more active metal such as zinc, aluminum, or magnesium. It then becomes a cathode where oxygen is reduced rather than an anode where iron is oxidized. The other metal acts as a sacrificial anode, which corrodes instead of iron.
An electric current flows between the two metals because of the difference in their activities; this results in the corrosion of the more active metal and thus iron remains protected. Taking the example of magnesium as the more active metal, the reactions are as follows.
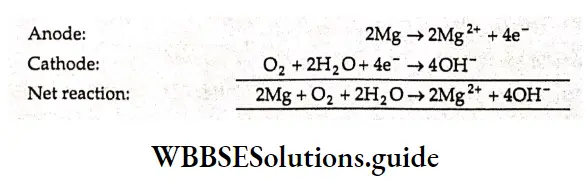
The active metal is slowly consumed and must be replaced periodically. This method of protection is used for large steel structures such as pipelines, storage tanks, bridges, and ships.
Batteries
Batteries are cells connected together so as to make maximum use of the reaction occurring in the cell and hence deliver a large current. Cells are of two types—primary and secondary.
Primary cells
Cells or batteries that cannot be recharged or become dead after use over a period of time are called primary cells or batteries. Examples include the Daniell cell and batteries used for torches, toys, etc.
A dry cell is a commonly used primary cell. Dry-cell batteries are used to power flashlights, radios, and other portable electronic devices. These are called ‘dry’ cells as the electrolyte inside them is a solid, in fact a paste. Since this type of cell was invented by the French engineer Georges Leclanche, it is also called a Leclanche cell.
The cell consists of a zinc container serving as an anode mid a graphite rod as the cathode. The graphite rod is surrounded by a moist mixture of ammonium chloride, manganese dioxide, zinc chloride and carbon. A porous paper liner serves as a separator of the two compartments and also as a salt bridge. When the cell is put to use, the zinc anode is oxidised forming zinc ions and electrons which flow through the external circuit to the cathode and reduce manganese dioxide at the cathode.
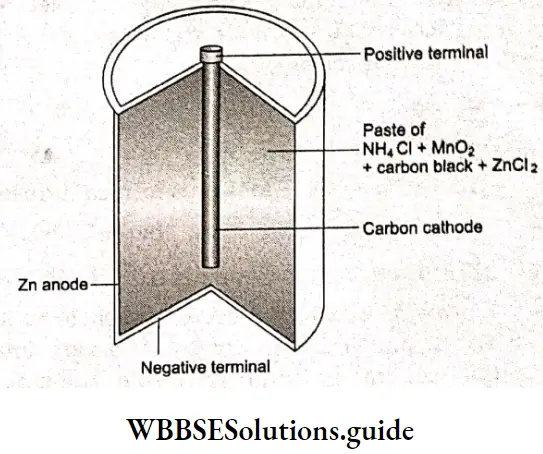
The reactions are:
anode: \(\mathrm{Zn}+2 \mathrm{NH}_4 \mathrm{Cl} \rightarrow 2 \mathrm{Zn}\left(\mathrm{NH}_3\right)_2 \mathrm{Cl}_2+2 \mathrm{H}^{+}+2 \mathrm{e}^{-} \text {or } \mathrm{Zn}(\mathrm{s}) \rightarrow \mathrm{Zn}^{2+}+2 \mathrm{e}^{-}\)
cathode: \(2 \mathrm{MnO}_2+2 \mathrm{e}^{-}+2 \mathrm{H}^{+} \rightarrow \mathrm{Mn}_2 \mathrm{O}_3+\mathrm{H}_2 \mathrm{O}\)
A cell commonly used in watches and also in pacemakers is the mercury cell. It consists of a zinc anode and a cathode made of steel in contact with mercury(II) oxide (HgO) in an alkaline medium of KOH and Zn(OH)–,. Zinc is oxidized at the anode and HgO is reduced at the cathode. The anodic and cathodic reactions are as follows.
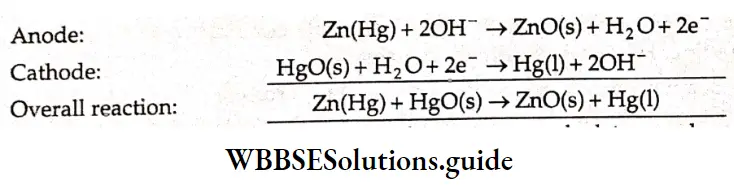
The small buttom-shaped batteries used in camars, calculators and watches are alkaline cells. Apart from these, dry cells have been replaced by longer lasting alkaline cells. An alkaline cell also consists of zinc and manages dioxide but the electrolyte contains potassium hydroxide instead of ammonium cloride. Under alkaline conditions, the anodic half reaction is
\(\mathrm{Zn}(\mathrm{s})+2 \mathrm{OH}^{-}(\mathrm{aq}) \rightarrow \mathrm{Zn}(\mathrm{OH})_2(\mathrm{~s})+2 \mathrm{e}^{-}\)while at the cathode MnOz is reduced to solid Mn2O3
\(2 \mathrm{MnO}_2(\mathrm{~s})+\mathrm{H}_2 \mathrm{O}(\mathrm{l})+2 \mathrm{e}^{-} \rightarrow \mathrm{Mn}_2 \mathrm{O}_3(\mathrm{~s})+2 \mathrm{OH}^{-}(\mathrm{aq})\)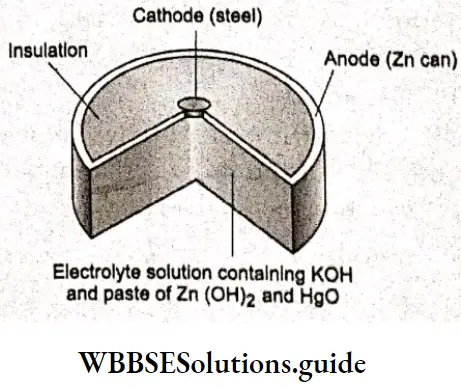
Alkaline cells have a greater shelf life (as Zn in the cell oxidizes slowly) and also last longer than dry cells as they have greater quantities of reactants than dry cells. Lists some commonly used batteries.
Some commonly used batteries and their applications:
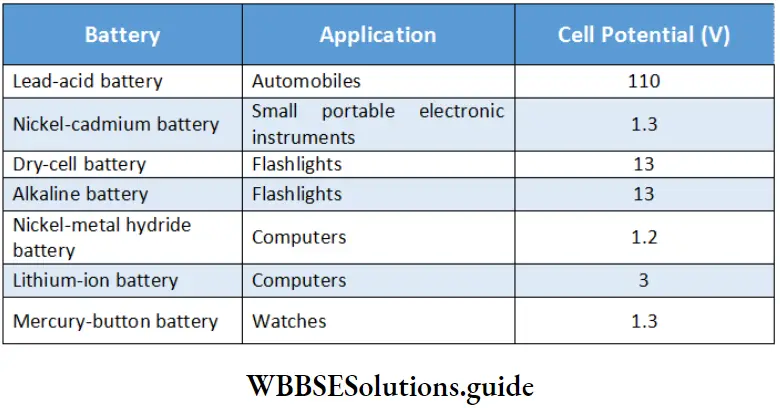
Secondary cells
Secondary cells are galvanic cells whose reactions can be reversed by the application of an external electric potential in a direction opposite to that of the discharge. This recharges the cell. The lead storage battery and nickel-cadmium (NICAD) cells are secondary cells.
The lead-storage or lead-acid battery is a dead one used in an automobile. The electrodes consist of lead-alloy grids; one set of grids is filled with lead(TV) oxide and the other with spongy lead metal The electrolyte used is dilute sulphuric add. When the battery delivers current, lead is oxidized to lead ions, these combine with the sulfate ions of sulphuric add and coat the lead electrode with insoluble lead sulfate.
The electrons released by the anode reaction flow through the external circuit to the cathode and reduce lead(IV) oxide to lead(II) ions, also forming water. These lead(2) ions also combine with sulfate ions forming lead sulfate and coating the cathode. The two half-reactions are
Anode: \(\mathrm{Pb}(\mathrm{s})+\mathrm{SO}_4^{2-}(\mathrm{aq}) \rightarrow \mathrm{PbSO}_4(\mathrm{~s})+2 \mathrm{e}^{-}[/laetx]
Cathode:
[latex]\mathrm{PbO}_2(\mathrm{~s})+4 \mathrm{H}_3 \mathrm{O}^{+}(\mathrm{aq})+\mathrm{SO}_4^{2-}(\mathrm{aq})+2 \mathrm{e}^{-} \rightarrow \mathrm{PbSO}_4(\mathrm{~s})+6 \mathrm{H}_2 \mathrm{O}(\mathrm{l})\)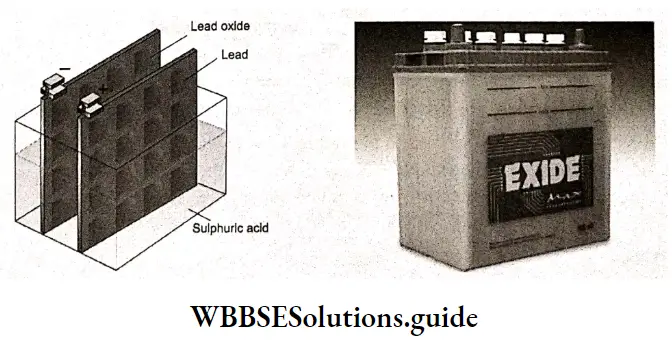
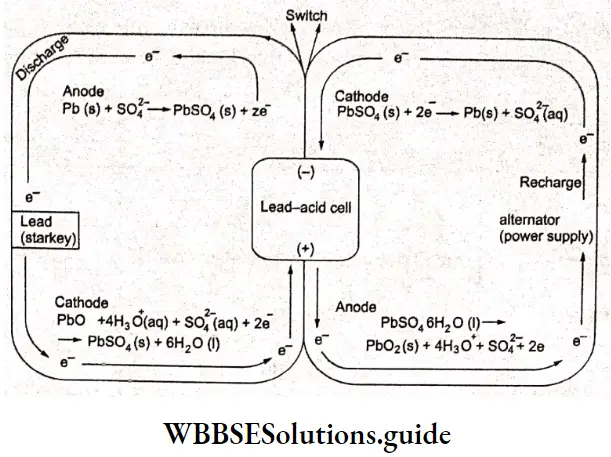
When used in a car, an alternator (power supply) recharges the battery by producing an external potential that pushes electrons in the reverse direction through the cell. The cell becomes an electrolytic cell during recharging. During recharging, the reactions at the cathode and anode are as follows.
Anode:
\(\mathrm{PbSO}_4(\mathrm{~s})+6 \mathrm{H}_2 \mathrm{O}(\mathrm{l}) \rightarrow \mathrm{PbO}_2(\mathrm{~s})+4 \mathrm{H}_3 \mathrm{O}^{+}+\mathrm{SO}_4^{2-}(\mathrm{aq})+2 \mathrm{e}^{-}\)Cathode:
\(\mathrm{PbSO}_4(\mathrm{~s})+2 \mathrm{e}^{-} \rightarrow \mathrm{Pb}(\mathrm{s})+\mathrm{SO}_4^{2-}(\mathrm{aq})\)The net reversible cell reaction in the lead storage battery is
\(\mathrm{Pb}+\mathrm{PbO}_2+4 \mathrm{H}_3 \mathrm{O}^{+}+2 \mathrm{SO}_4^{2-} \underset{\text { recharge }}{\stackrel{\text { discharge }}{\rightleftharpoons}} 2 \mathrm{PbSO}_4+6 \mathrm{H}_2 \mathrm{O}\)NICAD batteries contain nickel and cadmium electrodes. Cadmium acts as the anode and a hydroxide solution forms the electrolyte. NiO2 is reduced at the cathode.
The two half-reactions are:
anode:
\(\mathrm{Cd}(\mathrm{s})+2 \mathrm{OH}^{-}(\mathrm{aq}) \rightarrow \mathrm{Cd}(\mathrm{OH})_2(\mathrm{~s})+2 \mathrm{e}^{-}[/laetx]cathode:
[latex]\mathrm{NiO}_2(\mathrm{~s})+2 \mathrm{H}_2 \mathrm{O}(\mathrm{l})+2 \mathrm{e}^{-} \rightarrow \mathrm{Ni}(\mathrm{OH})_2(\mathrm{~s})+2 \mathrm{OH}^{-}\)
The net reaction is
\(\mathrm{Cd}+\mathrm{NiO}_2+2 \mathrm{H}_2 \mathrm{O} \underset{\text { recharge }}{\stackrel{\text { discharge }}{\rightleftharpoons}} \mathrm{Ni}(\mathrm{OH})_2+\mathrm{Cd}(\mathrm{OH})_2\)A solid coating of Cd(OH)2 forms around the anode and a solid coating of Ni(OH)2 forms around the cathode when the battery is discharged; these are converted back to the starting materials on recharging.
Earlier, Ni-Cd batteries were used in laptops but improvements were made and Ni-metal hydride (NiMH) batteries were developed. Here the cathode is NiO(OH) as in Ni-Cd batteries but the anode is made of a transition metal alloy such as LaNi5. The two electrodes are separated by a porous spacer containing an aqueous solution of KOH.
The reactions are
Cathode:
\(\mathrm{NiO}(\mathrm{OH})(\mathrm{s})+\mathrm{H}_2 \mathrm{O}(\mathrm{I})+\mathrm{e}^{-} \rightarrow \mathrm{Ni}(\mathrm{OH})_2(\mathrm{~s})+\mathrm{OH}^{-}(\mathrm{aq})\)Anode:
\(\mathrm{MH}(\mathrm{s})+\mathrm{OH}^{-} \rightarrow \mathrm{M}(\mathrm{s})+\mathrm{H}_2 \mathrm{O}(\mathrm{l})+\mathrm{e}^{-}\)
Lithium-Ion Batteries
High power demands by the late 1990s in high-speed processors, large video displays, and modem laptop computers led to the manufacture of lithium-ion batteries. These are based not on redox chemistry but on the concentration-driven migration of lithium ions. The anode is made of pure graphite and lithium ions are stored in it. The cathode is highly porous, made of transition-metal oxides such as MnOz, and forms a stable complex with lithium ions.
There is a high concentration of Li+ at the anode and a low concentration at the cathode. The migration of Li+ from the anode to the cathode (during discharge) is accompanied by that of electrons in an external circuit from the cathode to the anode. The electrolytes used in these batteries are solutions of lithium salts in non-aqueous solvents such as tetrahydrofuran, ethylene carbonate or propylene carbonate. Aqueous solutions cannot be used as electrolytes, because electrodes, particularly anodes, react with oxygen and water.
The electrode reactions are
Anode: \(\mathrm{Li}(\mathrm{s}) \rightarrow \mathrm{Li}^{+}+\mathrm{e}^{-}\)
Cathode: \(\mathrm{MnO}_2(\mathrm{~s})+\mathrm{Li}^{+}+\mathrm{e}^{-} \rightarrow \mathrm{LiMnO}_2(\mathrm{~s})\)
Fuel cells
In fuel cells, which are again galvanic cells, the electrode materials in the form of gases are supplied continuously to produce electricity. Such cells are used in space shuttles. The anode is a porous electrode with a catalyst such as finely divided platinum or palladium on its surface. Hydrogen gas is diffused through the anode.
The cathode is a porous electrode impregnated with cobalt oxide, platinum or silver as a catalyst. Oxygen gas is diffused through this electrode. The two electrodes are separated by a concentrated solution of sodium hydroxides or potassium hydroxide acting as an electrolyte. The two half-reactions are
Anode: \(2 \mathrm{H}_2+4 \mathrm{OH}^{-} \rightarrow 4 \mathrm{H}_2 \mathrm{O}+4 \mathrm{e}^{-} \quad E^{\ominus}=-0.828 \mathrm{~V}\)
Cathode: \(\mathrm{O}_2+2 \mathrm{H}_2 \mathrm{O}+4 \mathrm{e}^{-} \rightarrow 4 \mathrm{OH}^{-} \quad E^{\ominus}=0.401 \mathrm{~V}\)
The net cell reaction therefore leads to the formation of water as steam.
\(2 \mathrm{H}_2+\mathrm{O}_2 \rightarrow 2 \mathrm{H}_2 \mathrm{O}\)Methane or other hydrocarbons can also be used instead of hydrogen. Hydroxide ions formed at the cathode migrate through the electrolyte to the anode where they combine with hydrogen to form water.
When acidic electrolytes have used the reactions at the anode and cathode are anode:
Anode: \(\mathrm{H}_2(\mathrm{~g}) \rightarrow 2 \mathrm{H}^{+}+2 \mathrm{e}^{-} \quad E^\theta=0.00 \mathrm{~V}\)
Cathode: \(\mathrm{O}_2(\mathrm{~g})+4 \mathrm{H}^{+}(\mathrm{aq})+4 \mathrm{e}^{-} \rightarrow 2 \mathrm{H}_2 \mathrm{O}(\mathrm{l}) \quad E^{\Theta}=1.229 \mathrm{~V}\)
Fuel cells are promising alternative energy sources for automobiles.
Most of the fuel cells used in vehicles have a proton exchange membrane (PEM) between the two halves of the cell. Gas diffusers bring H2 and O2 to opposite sides of the membrane. At the anode side, H2 is oxidized to H+ ions. These ions migrate through die membrane to the cathodic side where they react with oxygen to produce water.
Another large-scale application of fuel cells is in power generation. Such cells have a lot of advantages over conventional thermal plants generating electricity. Their efficiency is 60 per cent more than that of thermal plants. In a thermal plant the liberated free energy of a chemical fuel (on burning) is used to first convert water into steam which is then used to drive a turbine. The turbine coupled with a generator produces electricity. A thermal plant causes pollution due to the burning of fossil fuels. On the other hand, fuel cells emit only water vapour—they do not pollute the air.
Electrochemistry Multiple-Choice Questions
Question 1. The flow of current in an electrolyte is due to the movement of
- Electrons
- Protons
- Ions
- None Of These
Answer: 3. Ions
Question 2. Metallic conductors conduct electricity through the movement of
- Ions
- Metal Atoms
- Free Electrons
- None Of These
Answer: 3. Free Electrons
Question 3. Which of the following substances does not conduct
- Crystalline NaCl
- Graphite
- CuSO, solution
- NaCl crystals with a defect
Answer: 1. Crystalline NaCl
Question 4. Conductance (G) is the reciprocal of electricity.
- Specific Resistance
- Current
- Resistance
- Concentration
Answer: 3. Resistance
Question 5. The specific conductance (K) or conductivity of any conducting material is defined as the
- Reciprocal Of Current
- Reciprocal Of Specific Resistance
- Reciprocal Of Resistance
- Product Of Specific Resistance And Current
Answer: 2. Reciprocal Of Specific Resistance
Question 6. The unit of specific conductance (x) is
- Ωcm
- Ω-1 cm
- -1 cm-1
- Ω cm-1
Answer: 3. -1 cm-1
Question 7. The cell constant of an electrochemical cell is given by
- \(\frac{k}{R}\)
- RK
- R2k
- \(\frac{R}{k}\)
Answer: 2. RK
Question 8. Calculate the molar conductivity of a 0.1 M H2SO, solution in S m2 mol-1 given that its specific conductance is 26 x 102 cm-1.
- 26×10-1
- 26×10-4
- 52×10-4
- 52×10-1
Answer: 2. 26×10-4
Question 9. On increasing the dilution, the specific conductance
- Increases
- Decreases
- Remains Constant
- None Of These
Answer: 2. Decreases
Question 10. Strong electrolytes are those which
- Dissolve Readily In Water
- Conduct Electricity
- Dissociate Into Ions Even At High Concentration
- Dissociate Into Ions At High Dilution
Answer: 3. Dissociate Into Ions Even At High Concentration
Question 11. Substances whose aqueous solutions are good conductors of electricity are called
- Strong Electrolytes
- Weak Electrolytes
- Nonelectrolytes
- Catalysts
Answer: 1. Strong Electrolytes
Question 12. The conductivity of an electrolyte depends on the
- Molecular Mass Of The Electrolyte
- The Boiling Point Of The Solvent
- Volume Of The Solvent
- Degree Of Ionization
Answer: 4. Degree Of Ionization
Question 13. In a galvanic cell
- Chemical Energy Is Converted Into Electrical Energy
- Electrical Energy Is Converted Into Chemical Energy
- The Cathode Is The Negative Terminal And The Anode Is The Positive Terminal
- None Of The Above
Answer: 1. Chemical Energy Is Converted Into Electrical Energy
Question 14. A salt bridge may contain
- A Saturated Solution Of Kcl And Agar-Agar
- A Saturated Solution Of Kno3 And Agar-Agar
- A Saturated Solution Of Nh4no3 And Agar-Agar
- All Of These
Answer: 4. All Of These
Question 15. The emf of a Daniell cell (given below) at 298 K is E.
\(\mathrm{Zn}\left|\mathrm{ZnSO}_4(0.01 \mathrm{M}) \| \mathrm{CuSO}_4(1.0 \mathrm{M})\right| \mathrm{Cu}\)When the concentration of ZnSO, is 1.0 M and that of CuSO4 is 0.01 M, the emf changes to E2. What is the relationship between E and E2?
- E1 >E2
- E1 < E2
- E1 =E2
- E2 = 0 ≠ E
Answer: 1. E1 >E2
Question 16. The Nearest equation
\(E_{\text {cell }}=E_{\text {cell }}^{\ominus}-\frac{R T}{n F} \ln Q\)
indicates that the equilibrium constant kc will be equal to Q when
- \(E=E^{\ominus}\)
- \(\frac{R T}{n F}=1\)
- E=0
- \(E^{\Theta}=1\)
Answer: 3. E=0
Question 17. The value of the reaction quotient Q for the cell
\(\mathrm{Zn}(\mathrm{s})\left|\mathrm{Zn}^{2+}(0.01 \mathrm{M}) \| \mathrm{Ag}^{+}(1.25 \mathrm{M})\right| \mathrm{Ag}(\mathrm{s})\) is
- 156
- 125
- 1.25×10-2
- 6.4 x 10-3
Answer: 4. 6.4×10-3
Question 18. For the cell reaction
\(\mathrm{Cu}^{2+}\left(c_1, \mathrm{aq}\right)+\mathrm{Zn}(\mathrm{s}) \rightleftharpoons \mathrm{Zn}^{2+}\left(c_2, \mathrm{aq}\right)+\mathrm{Cu}(\mathrm{s})\)
the change in free energy, AG, at a given temperature is a function of
- In c1
- \(\ln \left(\frac{c_2}{c_1}\right)\)
- In c2
- In (c1+c2)
Answer: 2. \(\ln \left(\frac{c_2}{c_1}\right)\)
Question 19. The standard reduction potentials of three metals A, B and C are +0.5 V, -3.0 V and -1.2 V respectively. Th arrangement of the metals, in order of their reducing power, is
- B>C>A
- A > B >C
- C>B>A
- A>C>B
Answer: 1. B>C>A
Question 20. For a cell reaction involving a two-electron change, the standard emf of the cell is found to be 0.295 V at 25°C. The equilibrium constant of the reaction at 25°C will be
- 1.0×10-10
- 29.5×10-2
- 10.0
- 1.0 x 1010
Answer: 4. 1.0×1010
Question 21. Given \(E_{\mathrm{Cu}^{2+} / \mathrm{Cu}}^{\ominus}=0.337 \mathrm{~V} \text { and } E_{\mathrm{Cu}^{2+} / \mathrm{Cu}^{+}}^{\Theta}=0.153 \mathrm{~V} \text {, calculate } E_{\mathrm{Cu}^{+} / \mathrm{Cu}^{\circ}}^{\Theta}\)
- 0.184 V
- 0.827 V
- 0.521 V
- 0.490 V
Answer: 1. 0.184 V
Question 22. A cell reaction is spontaneous when
- \(E_{\text {red }}^{\ominus} \text { is negative }\)
- \(E_{\text {red }}^{\ominus} \text { is positive }\)
- \(\Delta G^{\ominus} \text { is negative }\)
- \(\Delta G^{\ominus} \text { is positive }\)
Answer: 3. \(\Delta G^{\ominus} \text { is negative }\)
Question 23. The electrode potential for the following half-cell reactions are
\(\mathrm{Zn} \rightleftharpoons \mathrm{Zn}^{2+}+2 \mathrm{e} ; \quad E^{\ominus}=+0.76 \mathrm{~V}\) \(\mathrm{Fe} \rightleftharpoons \mathrm{Fe}^{2+}+2 \mathrm{e} ; \quad E^{\ominus}=+0.44 \mathrm{~V}\)The emf for the cell reaction
\(\mathrm{Fe}^{2+}+\mathrm{Zn} \longrightarrow \mathrm{Zn}^{2+}+\mathrm{Fe}\)- +0.32 V
- +1.20 V
- -0.32 V
- -1.20 V
Answer: 1. +0.32 V
Question 24. 9650 C of charge is passed through a solution of AgNO3. The mass of silver deposited on the cathode is (Atomic mass of Ag is 108)
- 1.08 g
- 10.8 g
- 21.6 g
- 100.0 g
Answer: 2. 10.8 g
Question 25. Three faradays of electricity are passed through molten Al2O3, an aqueous solution of CuSO, and molten NaCl taken in a separated electrolytic cell. The amount of Al, Cu, and Na deposited at the cathodes will be in the molar ratio of
- 1:2:3
- 3:2:1
- 1:15:3
- 1.5: 2:3
Answer: 3. 1:15:3
Question 26. Which of the following aqueous solutions remains neutral after electrolysis?
- CuSO4
- AgNO3
- K2SO4
- NaCl
Answer: 3. K2SO4
Question 27. How many faradays are required to reduce one mole of \(\mathrm{MnO}_4^{-} \text {to } \mathrm{Mn}^{2+}\)
- 1
- 2
- 3
- 5
Answer: 4. 5
Question 28. Aluminum oxide may be electrolyzed at 1000°C to = 96,500 coulombs). The cathode reaction is Al3++ 3e and would require furnished aluminum metal (at. mass = 27 amu, 1 faraday → AL To prepare 5.12 kg of aluminum metal by this method
- 5.49 × 10 C of electricity
- 5.49 x 10 C of electricity
- 1.83×107 C of electricity
- 5.49×101 C of electricity
Answer: 1. 5.49 × 107 C of electricity
Question 29. Copper containing zinc as an impurity is refined by electrolysis. The cathode and anode used are
Answer: 3.
Cathode: Pure copper
Anode: Impure copper
Question 30. Galvanized iron is
- Iron Coated With Tin
- Tin Coated With Iron
- Zinc Coated With Iron
- Iron Coated With Zinc
Answer: 4. Iron Coated With Zinc
Question 31. Rusting of iron is
- A Decomposition Process
- A Photochemical Process
- An Electrochemical Process
- A Reduction Process
Answer: 3. An Electrochemical Process
Question 32. In the rusting of iron, which of the following cell reactions occurs at the cathode?
- Fe2+/Fe
- O2/H2O
- Fe3+/Fe2+
- Fe/Fe3+
Answer: 3. Fe3+/Fe2+
Question 33. In a lead storage battery
- Pb is oxidized to PbSO, at the anode
- PbO2 is reduced to PbSO, at the cathode
- both electrodes are immersed in the same aqueous solution of H2SO4
- All The Above Are True
Answer: 4. All The Above Are True
Question 34. Which of the following statements is correct in the context of a battery?
- It is an electrochemical cell.
- It is used as a source of energy.
- The stored energy is released during the redox reaction.
- All of these
Answer: 4. All of these
Question 35. Which of the following reactions is used to make a fuel cell?
- Cd(s) + 2Ni(OH)3(s) → CdO(S) + 2Ni(OH)2(s) + H2O(1)
- Pb(s) + PbO2(s) + 2H2SO4(aq) → 2PbSO4 + 2H2O(1)
- 2H2(g) + O2(g) → 2H2O(1)
- 2Fe(s) + O2(g) + 4H2(aq) → 2Fe2+(aq) + 2H2O(1)
Answer: 3. 2H2(g) + O2(g) → 2H2O(1)